A technical comparison of NVIDIA data center GPUs. Explore detailed specs for Blackwell, Hopper & Ampere series (GB200, H100, A100, L40S) including FLOPS & NVLi

341 articles

A technical comparison of NVIDIA data center GPUs. Explore detailed specs for Blackwell, Hopper & Ampere series (GB200, H100, A100, L40S) including FLOPS & NVLi

Explore the exponential rise in AI compute demand in biotech. This 2025 report analyzes key statistics, infrastructure needs, and trends in drug discovery and g

Explore the AIME 2025 benchmark, a key test for AI mathematical reasoning. See how models like GPT-5 score over 94% and compare LLM performance on Olympiad-leve
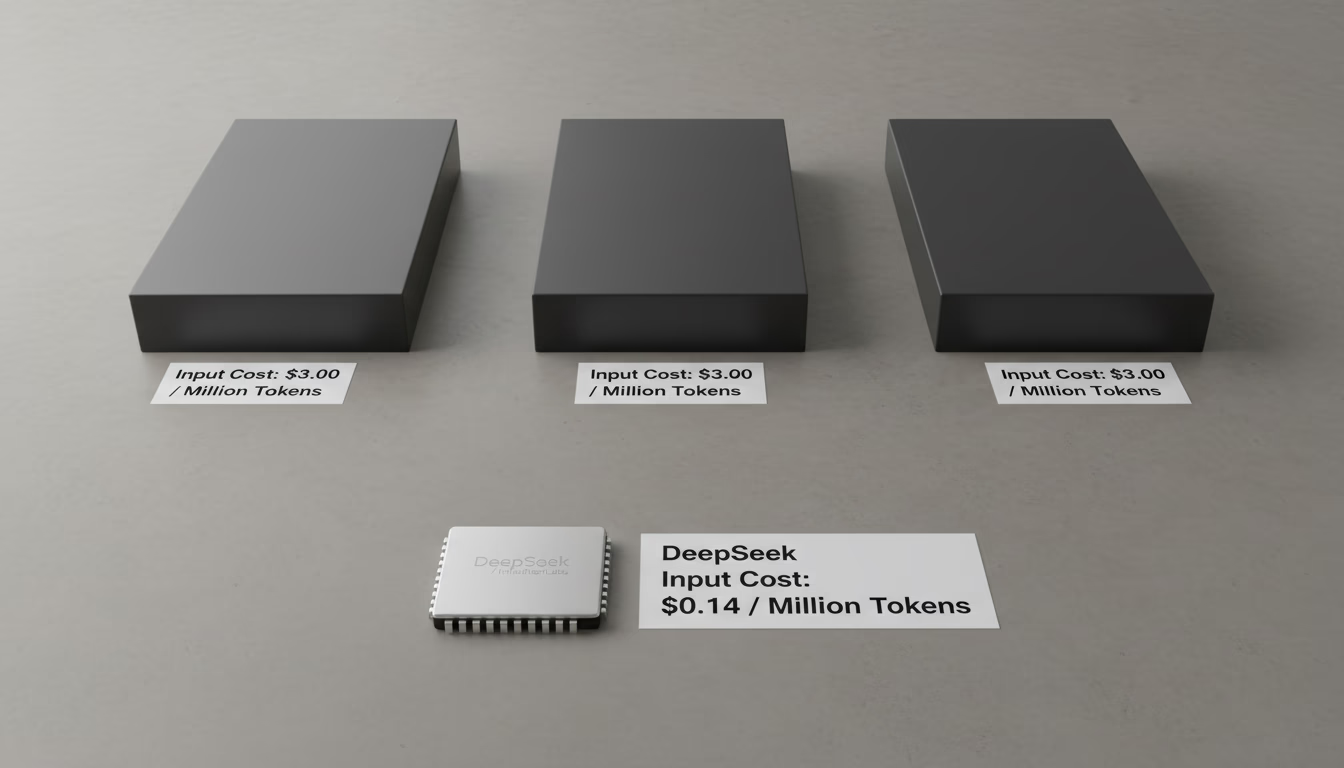
Learn why DeepSeek's AI inference is up to 50x cheaper than competitors. This analysis covers its Mixture-of-Experts (MoE) architecture and pricing strategy.
An expert guide to the GPQA-Diamond benchmark, a set of Google-proof questions testing AI on graduate-level scientific reasoning. Learn its purpose and design.

A technical guide to building a HIPAA-compliant OCR pipeline for healthcare. Learn key security controls, PHI handling, encryption, and cloud architecture.

An educational guide to HPC in life sciences. We review top lab IT specialists and solutions for genomics, drug discovery, and bioinformatics data analysis.

Learn what NVIDIA BioNeMo is and how it accelerates drug discovery. This guide explains its components, models, and deployment for generative AI in biopharma.

Learn what defines top Veeva experts in the life sciences industry. This guide profiles leading consultants and partners for Veeva Vault & CRM implementations.

Learn the strict physical requirements for deploying the NVIDIA HGX platform. This guide covers power, cooling, rack design, and floor loading for AI data cente

Analyze the leading data center providers for private AI solutions. This guide compares on-prem and hybrid infrastructure from AWS, Azure, HPE, Dell, and others

An in-depth analysis of ChatGPT Atlas, OpenAI's AI browser. Learn about its features like agent mode, strategic goals, and impact on traditional web search.

Get a complete 2025 analysis of Clinical Trial Management System (CTMS) software. This guide compares top vendors, core features, and eClinical market trends.

An analysis of how DocuSign is used for electronic signatures in pharma and life sciences. Learn how it can meet FDA 21 CFR Part 11 compliance with its modules.

Learn about Lorenz docuBridge, a key eCTD publishing software for biotech. This guide covers its features, editions, and use by regulators for regulatory submis

A comprehensive review of the NVIDIA DGX Spark. Explore its specs, performance benchmarks, price, and the consensus on its value for local AI development vs alt

Learn about Dassault Systèmes' QUMAS EDMS for life sciences. This guide covers its features, history, and use cases for GxP and 21 CFR Part 11 compliance.

An educational analysis of the remote inspection software market for life sciences. Learn about market size, growth drivers, QMS vendors, and PSC's ACE Inspecti

An in-depth analysis of Veeva AI Agents, the agentic AI integrated into the Veeva Vault platform for life sciences. Learn about its architecture, use cases, and

An in-depth 2025 analysis of AI accelerators from Cerebras, SambaNova, and Groq. Compare their unique chip architectures, funding, and performance for AI worklo

An in-depth analysis of the top 10 open source chatbot platforms for local deployment. Compare features, adoption data, and use cases for Rasa, Botpress, and mo

Compare on-prem AI infrastructure from Dell, HPE, Lenovo, Supermicro & Cisco. Analyze NVIDIA GB200/NVL72 hardware specs, cooling, software, and performance.
Learn about NVIDIA NVLink, a high-speed GPU interconnect designed to overcome PCIe bottlenecks. This guide explains its architecture, bandwidth, and impact on A

Learn about Innodisk APEX AI servers for running local AI models. This technical analysis covers hardware, specs, and why on-premise LLMs are vital for privacy.

An educational guide to enterprise LLM inference hardware. Compare NVIDIA & AMD GPUs with specialized AI accelerators for running powerful LLMs on-premises.

An in-depth analysis of the HMMT25 AI benchmark for testing advanced mathematical reasoning in LLMs. See how models like Grok-4 perform on complex problems.

Explore DeepSeek-OCR, an AI system that uses optical compression to process long documents. Learn how its vision-based approach solves long-context limits in LL
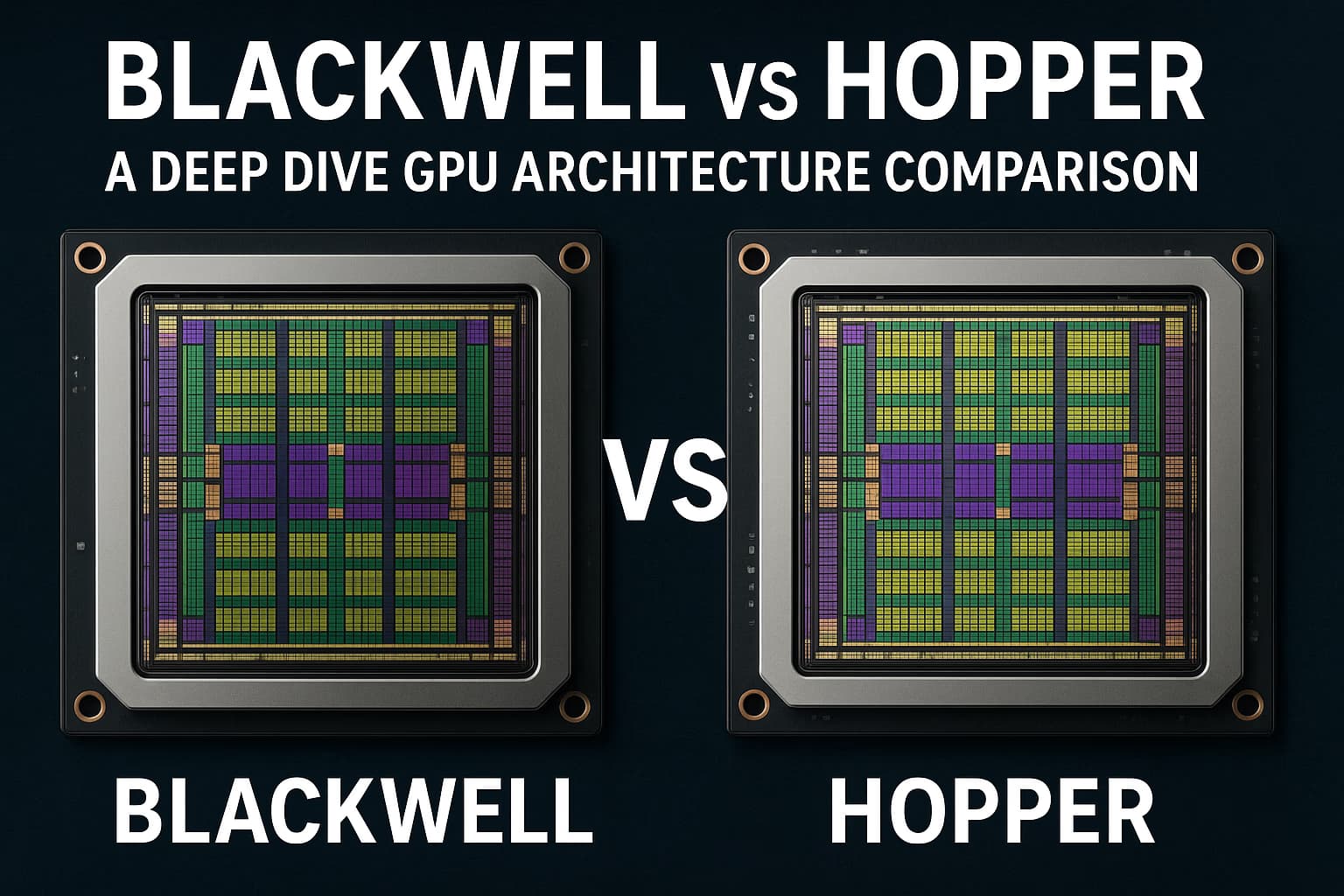
Explore the Nvidia Blackwell vs Hopper GPU architectures. Learn the key technical differences in tensor cores (FP4/FP6), memory, and performance for AI vs HPC.
Explore a data-driven comparison of the AI boom and the dot-com bubble. We analyze key metrics like valuations, profitability, and VC funding to see if this is
Explore the key differences between an AI engineer and a software engineer. Learn about their distinct skills, responsibilities, and core focus on ML vs. logic.

An in-depth analysis of Cognizant's RapidPro tool for Veeva Vault. Learn how this accelerator streamlines GxP data migration into the Veeva QualityDocs platform

Compare Zapier vs n8n for AI workflow automation. This in-depth report covers key differences in pricing, integrations, self-hosting, and AI capabilities.

Learn what context engineering is and how it improves AI and LLM reliability. This guide explains key techniques like RAG and why this is the next step past pro
Agentic AI projects face high failure rates. Learn the challenges of multi-agent workflows and why durable orchestration with Temporal.io is vital for reliabili

Get a detailed 2025 salary guide for a Medical Data Annotation Specialist. Learn about compensation in pharma & biotech, including US and UK pay benchmarks.

An in-depth guide to pharmaceutical MES and MOM software. Compare top vendors, understand cGMP compliance, and learn how systems enable electronic batch records

Learn how Reinforcement Learning from AI Feedback (RLAIF) reduces medical AI annotation costs. This guide covers the RLAIF method, its benefits over RLHF, and u
Explore the technical architecture of RLHF for drug discovery. Learn how reward models and policy optimization align generative AI with expert chemist feedback.

Build a safe and reliable clinical LLM using an RLHF pipeline. This guide covers the architecture, SFT, reward modeling, and AI alignment for healthcare.
An in-depth analysis of RLHF platforms for biotech. Compare Scale AI, Labelbox, Appen, and in-house solutions on capabilities, cost, and HIPAA compliance.

Explore the 2025 market for medical data labeling. This guide covers market size, growth, and the regulatory landscape, including HIPAA and the new EU AI Act.

Explore the digital therapeutics (DTx) market. This analysis covers market size, growth trends, key drivers, regulatory frameworks, and future outlook for 2025.

Learn about the EU AI Act's impact on pharma. This guide explains the risk-based approach, high-risk AI rules, and compliance steps with a free flowchart & SOP

Explore 2025 data on AI adoption in U.S. hospitals. This report covers key statistics, use cases like sepsis detection, EHR integration, and adoption disparitie

A technical comparison of Databricks vs. Snowflake for life sciences. Explore the lakehouse and cloud data platform for genomics, clinical data, and AI/ML workl

An analysis of GLM-4.6, the leading open-source coding model. Compare its benchmarks against Anthropic's Sonnet and OpenAI's GPT-5, and learn its hardware needs

An in-depth 2025 analysis of the leading medical imaging and digital pathology AI vendors. Covers market size, deep learning trends, and top company profiles.

Our 2025 report analyzes the critical AI skills gap in the pharmaceutical industry, detailing the talent shortage with data and exploring key solutions.

An in-depth guide to IT system assessment in pharma M&A due diligence. Explore key review areas like infrastructure, data, security, and compliance to avoid cos

Explore a detailed cost-benefit analysis of RTSM implementation in clinical trials. Learn how RTSM systems reduce drug waste by 15-30% and save millions.

Learn how target trial emulation provides a structured framework for drawing causal inference from real-world evidence (RWE) to support healthcare decisions.

Explore biotech salary trends across global regions. This data-driven analysis compares pay in top hubs like the US & Switzerland and details cost-of-living imp

In-depth Epic vs Cerner AI analysis. Compare how each EHR leader uses generative AI, predictive analytics, and voice tools to improve clinical workflows.

Learn the GAMP 5 software categories for computerized system validation. This guide explains each category with examples and its role in a risk-based approach.

A comprehensive guide to lab equipment qualification (IQ/OQ/PQ). Learn the process, regulatory standards, and how automation streamlines validation and complian

An in-depth review of Certara's drug development software suite for MIDD. Learn about Phoenix WinNonlin for PK/PD, the Simcyp PBPK Simulator, and QSP tools.
Learn the key differences between GAMP 4 and GAMP 5. This guide for pharma IT covers the shift to risk-based validation and provides a clear migration path.

Explore a data-driven profile of Peter Gassner, the founder of Veeva Systems. Learn about his career, Veeva's vertical SaaS strategy, and its growth.

Learn about Japan's PMDA regulatory pathways for drug approval under the PMD Act. This guide covers standard and expedited options like Sakigake and Orphan Drug

Understand the ICH M7 guideline for assessing and controlling mutagenic impurities in pharmaceuticals using in silico (Q)SAR software, TTC, and risk-based metho

A detailed analysis of the global pharmaceutical market, forecast to reach $1.6T by 2025. Explore key growth drivers, therapy areas, and industry challenges.

An in-depth study of pharmacy management systems. Explore key software features for dispensing, inventory, and billing, and compare top vendors in the market.

Learn about Patient-Reported Outcomes (PRO) systems in clinical trials. This guide covers ePRO data collection, PROMs, FDA regulatory guidelines, and challenges

An educational overview of AI-assisted surgery, profiling top companies like Intuitive Surgical, Medtronic, and J&J. Learn about robotic platforms and market da
This analysis examines the 2019 Veeva Crossix acquisition, detailing the integration of patient data with Veeva CRM and its impact on pharma marketing analytics

An in-depth analysis of the ICH E6(R3) Good Clinical Practice guidelines for 2025. Explore key changes in Quality by Design, data governance, and decentralized

An in-depth guide to the ICH Q10 model for Pharmaceutical Quality Systems (PQS). Explore its core elements, lifecycle approach, and integration with GMP regulat

Learn about synthetic data in pharmaceutical research. This guide covers acceptance criteria like fidelity, utility, and privacy for clinical and pharmacovigila

An overview of value-based contracting (VBC) in pharmaceuticals, where drug prices are tied to patient outcomes. Learn about models, challenges, and examples.
Compare Veeva vs Salesforce for life sciences CRM. This analysis covers features, GxP compliance, and the strategic shift to Veeva Vault vs Salesforce Life Scie

A detailed guide for IT on 21 CFR Part 11. Learn the FDA's rules for electronic records & signatures, including system validation, audit trails, & data integrit

Learn best practices for writing an effective FDA 483 response. This guide covers how to address inspectional observations and implement CAPAs to avoid a Warnin

An analysis of the 2025 surge in new drug manufacturing plants from Eli Lilly, AstraZeneca & more. Learn about the key drivers: supply chain & geopolitics.

An educational guide to pharmaceutical licensing deals. Learn key deal structures, including co-development, and financial terms like upfront payments, mileston

A detailed profile of Cognition Therapeutics (CGTX) analyzing its pipeline, financials, and lead drug CT1812, a sigma-2 modulator for Alzheimer's and DLB.

An educational guide to Veeva Vault certification. Learn about training paths for Business & System Administrators, exam costs, maintenance fees, and career ROI

Learn about HCP marketing in the pharmaceutical industry. This guide covers strategies, digital channels, omnichannel engagement, and key pharma regulations.

Explore Salesforce Health Cloud for biopharma. This in-depth guide covers its core CRM capabilities for patient engagement, clinical trials, and HCP relationshi

Learn the key updates in ISPE GAMP 5 Second Edition. Our guide covers the new focus on critical thinking, agile validation, cloud services, and CSA for GxP syst

An in-depth analysis of Merck's GPTeal, a secure generative AI platform. Learn how it uses LLMs to accelerate pharmaceutical R&D and boost productivity.

An in-depth case study of AstraZeneca's ChatGPT and generative AI implementation. Analyze its enterprise strategy, use cases in pharma R&D, and results.

Learn the end-to-end drug development pipeline, from initial drug discovery and preclinical research to Phase I-IV clinical trials and final FDA approval.

Explore top MS in AI for Drug Development programs for 2025. This guide reviews curricula, career prospects, and leading universities like UCSF and Maryland.

Analysis of top Learning Management Systems (LMS) for the biotech and life sciences industry. Compare validated platforms for GxP & 21 CFR Part 11 compliance.

Learn how to run private LLM inference for biotech. This guide covers on-premise deployment for data privacy, security, HIPAA compliance, cost analysis, and use

A comprehensive guide to Veeva Vault's 2025 platform updates. Learn about key features in 25R1, 25R2, & 25R3, including Action Triggers, Process Monitor & Veeva

Explore Bespoke Labs' specialized open-source LLMs. Learn how MiniChart (charts), MiniCheck (fact-checking), & OpenThinker (reasoning) are fine-tuned for specif

Learn about OpenAI's Stargate project, a $500B AI infrastructure initiative. This guide covers its datacenter locations, partners, technology, and global expans

See the 2025 list of the most expensive drugs in the USA, led by multi-million dollar gene therapies like Lenmeldy & Elevidys for rare diseases. Learn why price

Learn how spec-driven development (SDD) and GitHub's Spec Kit improve AI code generation. This guide explains the four-phase process for creating reliable softw

A summary of OpenAI DevDay 2025 announcements. Learn about new models like GPT-5 Pro and Sora 2, developer SDKs, and ChatGPT's evolution into an AI platform.

An overview of Anthropic's Claude Sonnet 4.5 model, its performance benchmarks, and new Claude Code 2.0 features like checkpoints, subagents, and IDE integratio

Learn how the FDA's Center for Veterinary Medicine (CVM) regulates animal drugs using safety and efficacy standards similar to human drugs, but with key differe

An index of open-source LIMS with details on each system's license, technology stack, and intended use for clinical, research, and biobanking labs.

A guide to the top 10 specialty pharmacy management platforms. We compare systems from WellSky & McKesson to new AI tools for prior authorization & patient care

A review of top AI chatbots in healthcare. Learn how NLP and LLMs power tools for medical triage, symptom checking, and mental health support like CBT.

An overview of IBM's Granite 4.0 LLM, detailing its hybrid Mamba/Transformer design, efficiency benefits, and applications for healthcare AI and data privacy.

Analysis of AI's role in hospital operations for 2025, covering automated documentation, workflow efficiency, and reduced physician burnout with new data and ca

This article examines the OpenAI-AMD strategic partnership, detailing the GPU supply terms, stock warrants, and the advantages and risks for AI hardware strateg

An overview of a Clinical Outcomes Management System (COMS) for long-term care. Learn how evidence-based protocols and clinical decision support improve patient

Learn about AI company Cohere, its enterprise focus, and its Command family of LLMs. This article details its history, key personnel, and model performance.

A data-driven analysis of the US biotech job market in 2025. Learn about employment trends, the skills gap, top geographic hubs, and the industry's future outlo

Learn how to create apps for ChatGPT in 2025. This guide explains the OpenAI Apps SDK, the GPT Store, custom GPTs, and provides a step-by-step developer workflo

This article profiles five leading technology companies in healthcare AI. It examines their key solutions for data analytics, patient engagement, and life scien

Learn best practices to evaluate CRO costs for your clinical trial budget. This guide covers RFPs, comparing pricing models, and analyzing CRO proposal details.

A detailed overview of IQVIA's technology solutions for life sciences. Learn about platforms for data management, commercial engagement, analytics, and RWE.

Learn how to build robust LLM evaluation frameworks for biotech. This guide covers key metrics, biomedical benchmarks (BLUE, BLURB), and methods for ensuring ac

An overview of Next Best Action (NBA) in pharma. Learn how this AI strategy uses data to suggest optimal engagement actions for healthcare professionals.

An analysis of China's open-source LLM landscape in 2025. Covers key models like Qwen, Ernie, and GLM from major tech firms and leading AI startups.

Learn the hardware requirements for running OpenAI's GPT-OSS-20B model locally. This guide covers GPU VRAM, CPU, system RAM, and other key components.

An examination of the five key technical innovations behind ChatGPT, from the Transformer architecture and pretraining to RLHF, hardware, and tokenization.

An analysis of leading software for molecular modeling and simulation. Learn about features, theoretical methods (MD, QM), performance, and use cases.

Learn about Apache Airflow's core architecture, including DAGs, schedulers, and executors, and its application for building data workflows in life sciences.

An in-depth technical comparison of five cheminformatics platforms, evaluating features like SAR/QSAR, ADMET prediction, and chemical library management.

Examines the 2025 AI regulatory frameworks for biopharma in the U.S., EU, U.K., and Canada. Details compliance obligations for GenAI, GxP, and SaMD.
Learn the architectural differences between event sourcing and queue-based systems. This article covers immutable event logs, data traceability, and replayability.
Learn about Mixture of Experts (MoE) models, a neural network architecture using specialized experts and a gating mechanism to efficiently scale computation.

An educational overview of hospice EMR systems, including essential features for documentation, scheduling, billing, and ensuring HIPAA compliance.

An overview of GAMP 5 guidelines for validating computerized systems. Explains the risk-based approach, system lifecycle, and updates for AI and cloud tech.

Learn about the specialized software tools used across the drug development lifecycle, from discovery and preclinical research to manufacturing and commercialization.

An analysis of Regulatory Information Management (RIM) systems, covering their role in life science compliance, eCTD submissions, and enterprise integration.

This article provides a technical overview of pharmacovigilance software, detailing core functions, regulatory compliance, and a comparison of leading platforms.

Learn what Policy as Code (PaC) is and why it is used in healthcare to automate enforcement of security, privacy, and compliance regulations.

Examines how to apply Git version control workflows to satisfy FDA compliance, covering traceability, audit trails, and standards like 21 CFR Part 11 & IEC 62304.

Learn about FHIR (Fast Healthcare Interoperability Resources), the modern HL7 standard for exchanging health data using web APIs and self-contained resources.

An overview of AI agents in pharmacovigilance (PV), detailing their application in processing safety data and managing the increasing volume of ICSRs.

An overview of the Centers for Medicare & Medicaid Services (CMS) definition for TPMOs and their role within the "chain of enrollment" for Medicare plans.

An overview of the IEC 62304 standard for medical device software. This guide explains its purpose, scope, and required life cycle processes for safety.

An analysis of top ERP systems for the pharmaceutical sector, evaluated on regulatory compliance (21 CFR Part 11), batch traceability, and serialization.

Examine the role of AI in Health Information Exchange (HIE), focusing on data standardization, security, predictive analytics, and ethical considerations.

This article explains the core functions of LIMS for sample management, data integrity, and regulatory compliance, and provides a comparative analysis of top systems.

Learn methods for integrating ChatGPT with private enterprise data using Microsoft Azure. Covers security controls, compliance, and the Azure OpenAI Service.

Learn about the FDA Adverse Event Reporting System (FAERS), a database for post-marketing drug safety surveillance and detecting new adverse event signals.

Learn the official definition of Software as a Medical Device (SaMD) from the IMDRF. Understand its scope as standalone software and its regulatory status.

Learn about Clinical Decision Support (CDS) systems, from early rule-based expert systems to modern data-driven models powered by artificial intelligence.

This article defines open-source LIMS and details its core functions, such as sample registration, data collection, quality control, and workflow management.

This article provides a comprehensive review of global Health Management Information Systems (HMIS), detailing their function, software types, and implementation.

An overview of the healthcare revenue cycle, a financial process covering all administrative and clinical functions from patient scheduling to payment collection.

An explanation of Clinical Data Management (CDM) and its function in research. Learn how CDM ensures high-quality, reliable data for clinical trials.

An overview of key researchers and industry leaders in the US applying generative AI models to pharmaceutical R&D, drug discovery, and biomarker research.

Learn about AHIP certification, the standard for Medicare agents. This guide explains the MFWA training, program requirements, costs, and its industry role.

An analysis of AI adoption trends in U.S. hospitals. Examines clinical and operational use cases, technology partnerships, and ethical considerations in healthcare.

Examines the top 10 programming languages by usage and popularity in the context of AI-assisted coding, analyzing AI tool support and ecosystem maturity.

An analysis of the 2025 healthcare administration job market. Examines key trends, demand drivers, worker shortages, and BLS employment projections.

An analysis of large language model (LLM) diagnostic performance on medical benchmarks compared to physicians, exploring accuracy, limitations, and implications.

Learn about mechanistic interpretability, a method to reverse-engineer AI models. This article explains how it uncovers causal mechanisms within neural networks.

An overview of pharmaceutical KPIs for quality, compliance, and operational excellence. Explores metrics for R&D, manufacturing, and regulatory standards.

An analysis of the core technologies and design philosophies of Wolfram Alpha (symbolic AI) and ChatGPT (generative AI), detailing their key differences.

This article explains how pill identifier software improves patient safety by accurately identifying medication using physical traits, preventing costly medical errors.

An educational profile of ZUSDURI, a mitomycin hydrogel for intravesical chemoablation of low-grade, intermediate-risk non-muscle invasive bladder cancer (NMIBC).

An analysis of the Medicare program in 2025, detailing the structure and the significant policy impacts of the One Big Beautiful Bill Act (P.L. 119-21).

Examine 2025 trends for Contract Sales Organizations in pharma. This analysis compares top providers and strategies for HCP engagement and outsourcing.

This article explains how AI tools can validate regulatory dossiers, catch technical errors before submission, and minimize rework for emerging biotech companies.

Examines the evolution of eCTD systems from specialized publishers for regulatory submissions to enterprise platforms that serve as a unified drug lifecycle archive.

This article examines the unique financial challenges facing regional hospitals and details the accounting practices essential for maintaining stability and compliance.

An overview of San Francisco Bay Area neuroscience firms. Examines companies in neurotechnology, BCIs, and neuropharmacology, detailing their focus and status.

An overview of AI in pharmacovigilance, detailing its use in signal detection, case processing automation, and managing regulatory & ethical considerations.

An overview of market access analytics, covering its definition, scope, and role in helping pharmaceutical firms navigate complex payer reimbursement models.

Learn to design, secure, and manage a HIPAA-compliant API. This guide covers HIPAA rules, technical best practices, and risk management for health data.

Examines the use of Veeva CRM across the pharmaceutical and life sciences sectors. Details companies using the platform, with evidence from job postings.

Learn how WORM (Write Once, Read Many) storage ensures data immutability and integrity for electronic records in the highly regulated biotechnology industry.

Examine the technical capabilities of GPT-5, its applications in life sciences and medicine, and the ethical considerations for its use in these fields.

Examines the key scientific journals and trade publications in the pharma and biotech industries, covering peer-review, access models, and target audience.

An educational guide to executing compliant pharmaceutical remarketing. Explores platform policies on Google, Meta, LinkedIn, and Reddit, plus data privacy.

An overview of AI applications in the pharmaceutical sector, from generative AI to ML. Explains key IT management challenges like data, compliance, and security.

Review a list of global online degrees and certificates in AI for pharmaceutical science. Compare programs by level, curriculum, cost, and duration.

An overview of OpenAI's open-weight GPT-OSS models. Examine their technical specifications, benchmark performance, and applications for reasoning in healthcare.

Examines how AI accelerates the pharmaceutical drug pipeline, reducing time to market. Learn about its impact on preclinical testing, clinical trials, and manufacturing.

Examines RegTech adoption challenges for emerging biotechs, focusing on cost and scalability. Explains flexible licensing and deferred-value models for compliance.

An explanation of active learning principles and their adaptation for Large Language Models (LLMs) using human-in-the-loop (HITL) feedback for model alignment.

This article examines strategies for integrating content management systems like Box and Veeva Vault to overcome information silos in regulated environments.

An overview of the pharmaceutical industry's history, from 19th-century origins to the current global market size, sales figures, and regional distribution.

An explanation of regulatory requirements for audit trails under 21 CFR Part 11 and EU Annex 11, covering automation strategies and data integrity measures.

An explanation of the Model Context Protocol (MCP) and its integration with Claude Code. Learn how MCP servers enable AI agents to perform internet searches.

An explanation of a single source of truth (SSOT) for the pharmaceutical industry. Learn how an SSOT integrates data across the drug lifecycle to ensure consistency.

An analysis of how CRM systems support medical device firms in managing HCP relationships, sales pipelines, and maintaining strict regulatory compliance.

An analysis of AI code assistants in large codebases. Evaluates Copilot, CodeWhisperer, and others on accuracy, context handling, security, and IDE integration.

This report details how pharmaceutical companies use mobile apps for patient support, medication adherence, and data collection, outlining strategies and challenges.

An overview of Reinforcement Learning (RL) and RLHF. Learn how RL uses reward functions and how RLHF incorporates human judgments to train AI agents.

Explore a comprehensive comparison of Tableau and Power BI, analyzing market share, features, pricing, and adoption trends for data professionals.

A technical review of dedicated OCR engines not based on LLMs. Examines computer vision and sequence modeling architectures, performance, and applications.

Explore how ERP systems integrate biotech R&D, manufacturing, and finance, ensuring GxP compliance and traceability. Covers major and niche vendor solutions.

A technical guide to Reinforcement Learning from Human Feedback (RLHF). This article covers its core concepts, training pipeline, and key alignment algorithms.

This article details AI applications in pharmaceutical business intelligence, covering drug discovery, clinical trials, supply chain, real-world evidence, and market intelligence.

Examines Kimi K2, a trillion-parameter open-weight LLM from Moonshot AI. Learn its technical details, development background, and strategic context.

This article explains Integrated Business Planning (IBP) as a strategic imperative for the pharmaceutical industry, detailing its role in unifying cross-functional plans and decision-making.

Explore 21 CFR Part 11 compliance for electronic records, signatures, and AI in GxP. Covers key elements, FDA guidance, and controls for data integrity and audit-ready systems.

This article defines Sales Force Effectiveness (SFE) in the MedTech industry, explaining its strategic importance for optimizing performance and achieving growth in a competitive market.

Explore key software needs, technology stacks, and specific tools like AI-driven drug design and cheminformatics in pharmaceutical software development.

Explore US pharmaceutical automation compliance, covering FDA regulations like cGMP & 21 CFR Part 11, Pharma 4.0 trends, challenges, and best practices.

Examines how an OpenAI AI system achieved a gold medal score at the 2025 IMO, detailing its performance, natural-language proofs, and AI reasoning ability.

This article explains pharmaceutical serialization software, its critical role in securing the drug supply chain, and adherence to regulations like DSCSA and FMD.

An analysis of AI applications for Veeva Systems in life sciences. Examines emerging consultancies, market trends, and use cases in regulatory and commercial ops.

Evaluate top 10 accounting/ERP solutions for pharmaceutical companies. Learn about features like regulatory compliance, batch tracking, and quality control systems.

Explore AWS cloud computing's role in life sciences for scalable data processing, HPC, and analytics. Learn how AWS facilitates innovation in biotech and pharma.

Learn how Power BI consultants apply business intelligence to pharmaceutical data, addressing regulatory needs, clinical trial insights, and RWE analysis.

Explore AI code assistants suitable for air-gapped, on-premises enterprise deployment. Understand infrastructure, security, and integration for highly regulated environments.

This article details AI's role in biotech sample management, covering traditional workflows, challenges, AI innovations, regulatory issues, and future outlook.

Explore clinical AI's role in patient care, decision-making, and medical data analysis. Learn about its applications in diagnosis, treatment, and outcome prediction, driven by tech advances.

Explore how Oracle Cloud CX supports customer experience in life sciences, encompassing CRM, marketing, and service applications for compliant engagement with HCPs and patients.

Explore Medidata Rave CTMS and EDC solutions, including their history, features, and real-world application in clinical trials. Learn about market standing and competitors.

Learn about 10 key AI innovations that optimize clinical trials, improving efficiency, reducing costs, enhancing patient safety, and speeding drug development.

Learn about key technical, regulatory, organizational, ethical, and financial barriers hindering AI adoption in life sciences, with emerging solutions.

Explore how generative AI is applied in mRNA vaccine development, using Moderna and Pfizer's COVID-19 vaccine as a case study to understand rapid immunization advancements.

Learn why new drug development takes over a decade, discussing the high attrition rates, extensive research, and regulatory hurdles involved in bringing medicines to market.

Learn how regulatory affairs ensures product compliance in health industries. Explore the fundamental role of AI and LLMs in modern regulatory processes.

Learn about the 2025 job market for Computer System Validation (CSV) professionals. Understand CSV's critical role in regulated industries for data integrity and patient safety.

Learn about regulatory compliance software in pharmaceuticals, covering GMP, GLP, GCP, FDA 21 CFR Part 11, and EU Annex 11 standards for data integrity.

This report analyzes Veeva Systems' US executive compensation, detailing CEO and CFO pay components sourced from SEC filings and crowdsourced data. Learn about salary, bonus, and equity.

Learn about Pharmaceutical Field Force Effectiveness (FFE), its definition, strategic importance for commercial goals, and key performance indicators.

Explore pharmaceutical commercial operations, covering essential activities from market research and marketing strategy to sales execution and product distribution after regulatory approval.

Explore ChatGPT as a Generative AI and Large Language Model. Learn its core GPT architecture, Transformer backbone, and how it processes language.

This guide details a structured approach for Generative AI adoption in life sciences, covering strategy, governance, technology, training, and ethical considerations.

Learn how NetSuite ERP modules support U.S. pharmaceutical manufacturing labs, covering GMP, FDA (21 CFR Part 11) compliance, and integrations for quality and production.

Compare GAMP 4 and GAMP 5. Learn key differences in their approaches to system validation, risk management, system classification, and integration of modern technologies.

Evaluate LMS platforms for life sciences. Understand key compliance requirements: FDA 21 CFR Part 11, GxP, electronic records, and audit trails for validated training.

Explore the distinct roles and benefits of Learning Management Systems (LMS), Digital Adoption Platforms (DAP), and Knowledge Management Systems (KMS) in life sciences.

Compares NetSuite and SAP ERP systems' technical capabilities and regulatory compliance features for pharmaceutical and life sciences companies.

Learn about leading pharmaceutical market intelligence firms, their data analysis methods, and services like drug pipeline tracking, sales forecasts, and regulatory insights.

This article lists 10 free generative AI courses for pharmaceutical professionals. Learn LLMs, prompt engineering, and AI applications in drug R&D.

Learn how ICD-10 codes, essential for healthcare data in EHRs, are transformed into numerical embedding vector spaces for machine learning and data science applications.

Explore the history and development of Anthropic's Claude 4 large language model, covering its evolution, key features, and advancements over prior versions.

This article compares the user interfaces of leading conversational AI tools like ChatGPT, Gemini, and Claude, detailing their design, features, and professional impact for business users.

Learn about 21 CFR Part 11, the FDA regulation for electronic records and signatures. This article covers its history, purpose, and significance in the pharmaceutical industry.

Explore Good Manufacturing Practice (GMP) in pharma. Learn its principles, global regulations, implementation, and compliance challenges for quality assurance.

This guide explains GAMP 5, its principles, and lifecycle for computerized system validation in pharma, covering regulatory compliance and real-world applications.

Learn what Computer System Validation (CSV) is, its crucial role in pharmaceutical and biotech compliance, ensuring data integrity and regulatory adherence for patient safety.
Compares leading AI OCR models and tools for PDF to structured text conversion. Examines open-source and commercial solutions, assessing capabilities, accuracy, and use cases.

This report details deploying LLMs on 24GB GPUs, covering model architectures, VRAM needs, and optimization methods for efficient local operation.

Learn about meta-prompting, an advanced technique using LLMs to generate, modify, and optimize their own prompts, enabling iterative and complex task execution.

Explore profiles of top global pharmaceutical thought leaders, including their backgrounds, speaking topics, notable engagements, and industry influence.

Learn to build a pharmaceutical manufacturing plant from concept through commissioning. This guide covers planning, regulatory compliance, design, equipment, quality, validation, and approval.

Explore how AI-powered computer vision is transforming pharmaceutical quality control. Review top vendors, applications, and trends in pharma QC automation.

How AI leaders like Amodei, Hassabis, and Huang are accelerating drug discovery, genomics, and life science breakthroughs—compressing decades into years.

Comprehensive guide to BI and dashboard tools for pharma: Power BI, Tableau, Qlik, Looker, Domo, compliance, pricing, and adoption in life sciences.

How Veeva Crossix and big data are transforming pharma marketing analytics: capabilities, compliance, case studies, and adoption trends in the U.S.

Guide to MarTech API integrations for pharma: CRM, marketing automation, analytics, consent, compliance, and best practices for unified digital engagement.

In-depth guide to U.S. pharmaceutical marketing regulations: FDA, FTC, Sunshine Act, anti-kickback, compliance, and IT obligations for pharma companies.
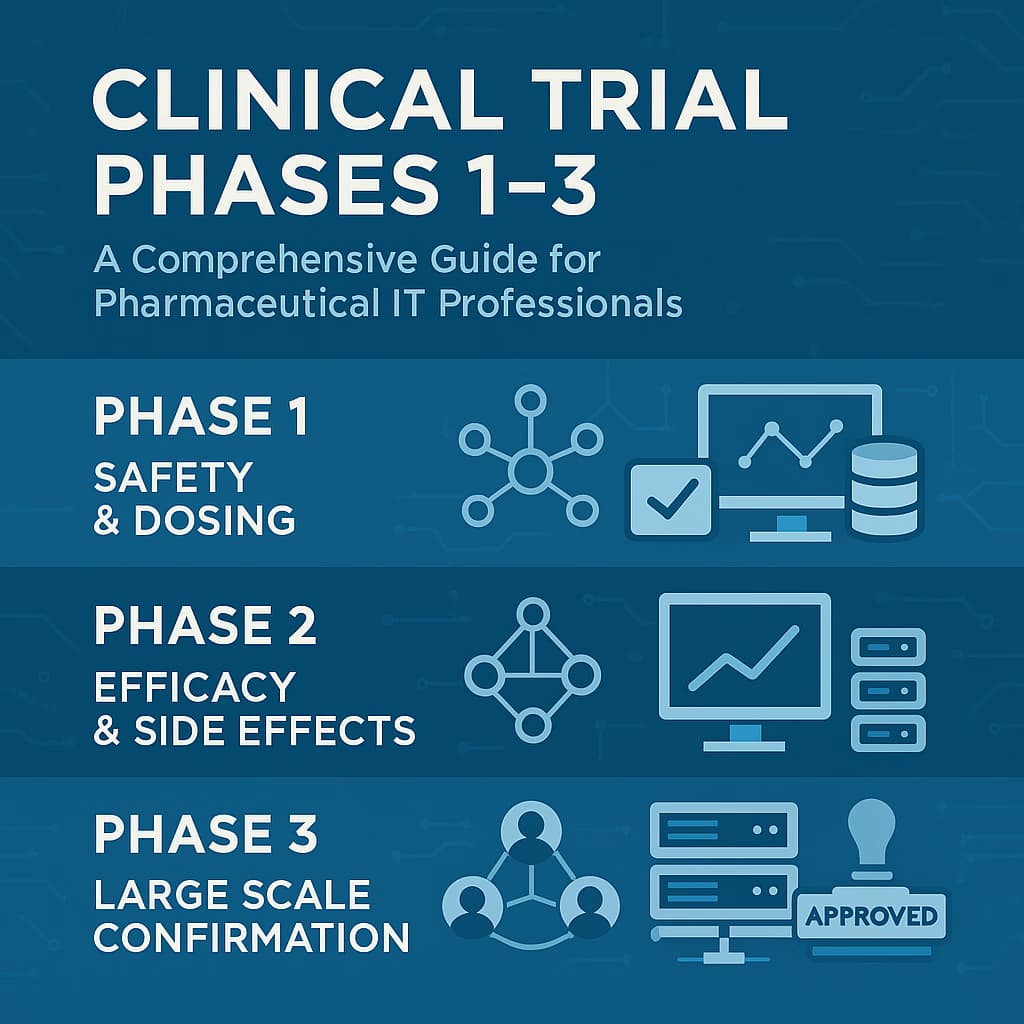
A practical guide to clinical trial phases 1–3 for pharma IT: objectives, compliance, costs, and IT infrastructure for modern drug development.

Guide to outsourcing HCP marketing in pharma: benefits, risks, industry trends, compliance, and how to manage third-party partnerships effectively.

How Veeva Site Connect streamlines sponsor–CRO–site collaboration: features, adoption, compliance, and comparison to Medidata and Oracle Clinical One.

Best practices for pharma teams to secure first meetings with HCPs: outreach tactics, channel comparison, overcoming gatekeepers, and expert tips for success.

A step-by-step career roadmap for pharma IT professionals: from Veeva admin to enterprise architect. Skills, certifications, salary benchmarks, and real-world examples.

Overview of major HCP data providers, U.S. compliance rules, and best practices for pharma IT. Includes vendor comparison, legal requirements, and governance tips.

How biotech and pharma companies use NetSuite for compliance: FDA 21 CFR Part 11, GxP, HIPAA, SOX, audit trails, e-signatures, and reporting best practices.

Evidence-based UX strategies for digital HCP engagement platforms in pharma: trends, challenges, best practices, and compliance for IT teams.
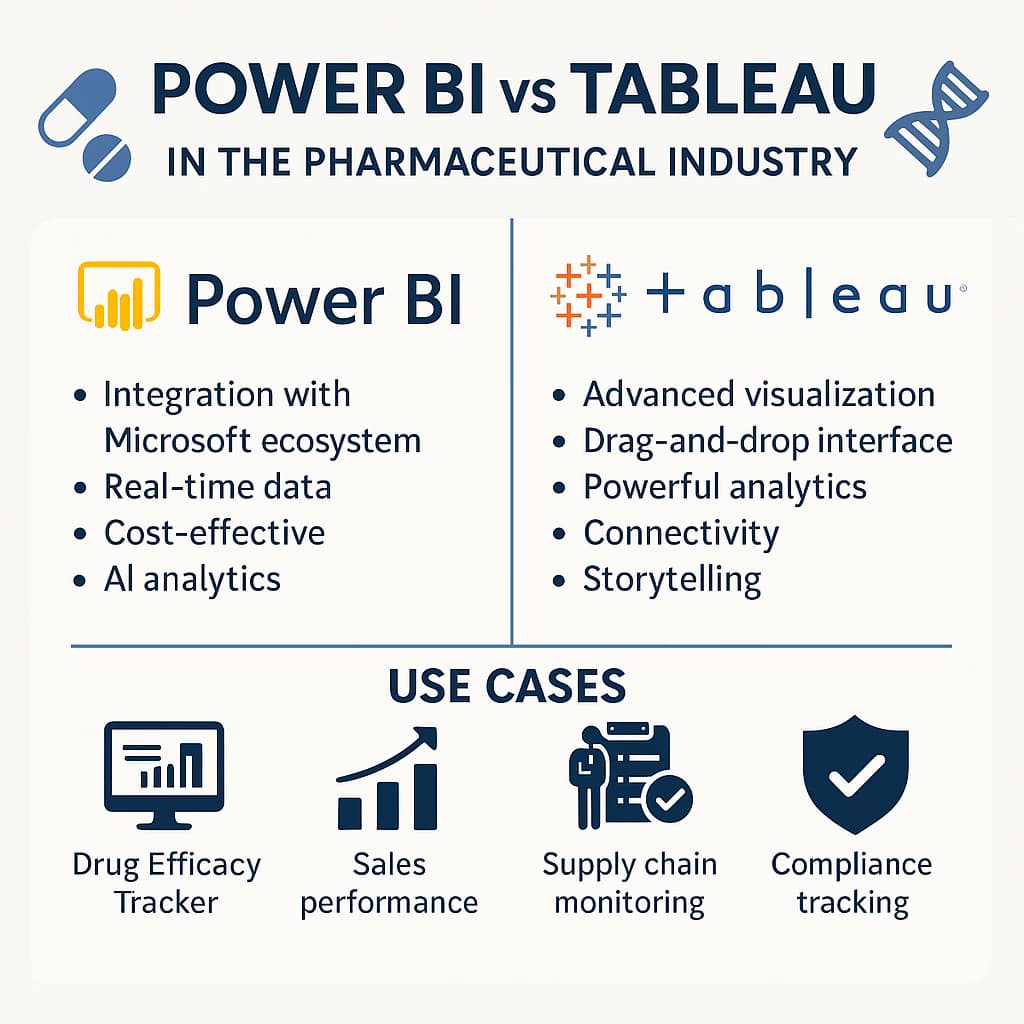
Comprehensive comparison of Power BI and Tableau for pharma: features, pricing, compliance, and use cases for IT and analytics teams.

A technical comparison of AI agents and AI workflows in pharmaceutical IT, with use cases, pros and cons, and adoption trends for U.S. pharma.

A technical overview of remote detailing in the pharmaceutical industry: adoption trends, technology platforms, compliance, and best practices.

A detailed survey of large language model benchmarks in life sciences, covering biomedical NLP, drug discovery, and genomics, with industry use cases and top model performance.

A comprehensive review of software solutions for optimizing pharmaceutical field sales routes, comparing CRM, SFA, and mapping tools for efficiency, compliance, and analytics.

A detailed comparison of Windsurf (Codeium), Cursor, and GitHub Copilot for enterprise software development in the pharmaceutical industry, focusing on security, compliance, and productivity.

A comprehensive guide to building and scaling Veeva Vault and CRM data pipelines for terabyte-scale datasets in the pharmaceutical industry, with a focus on compliance and performance.

An exploration of how artificial intelligence is revolutionizing drug development processes, from target identification to clinical trials, with focus on implementation strategies and success metrics.

An analysis of how artificial intelligence is transforming regulatory affairs in pharmaceuticals, from submission preparation to compliance monitoring and regulatory intelligence.

Step-by-step guide to all Veeva Vault login methods: username/password, SSO, mobile app, and external/partner access. Includes troubleshooting, FAQs, and application-specific notes.

A technical guide to developing customized CRM solutions for pharmaceutical companies using AI-assisted development tools, focusing on compliance, data security, and industry-specific requirements.

Detailed case studies examining successful implementations of Randomization and Trial Supply Management systems in U.S. clinical trials, highlighting best practices and measurable outcomes.

A comprehensive analysis of ChatGPT integration in life sciences, examining implementation strategies, regulatory compliance, and real-world applications across pharmaceutical research and development.

An in-depth analysis of the biotech ecosystem in the San Francisco Bay Area, examining key players, emerging startups, investment trends, and innovation clusters in 2025.

Exploring how modern RTSM solutions are evolving to improve patient experience in clinical trials, featuring innovative approaches to recruitment, engagement, and trial management.

A detailed comparison of key compliance frameworks in pharmaceutical IT, including FDA 21 CFR Part 11, GDPR, HIPAA, and GxP, with implementation strategies and best practices.
An evaluation of RAG systems' effectiveness in processing pharmaceutical documentation, analyzing accuracy, compliance adherence, and practical applications in drug development and clinical trials.

An in-depth comparison of cloud-based and on-premise Randomization and Trial Supply Management (RTSM) solutions, analyzing security, compliance, cost, and operational considerations for pharmaceutical companies.

A comprehensive analysis of the remote patient monitoring landscape in the US healthcare system, examining technological advances, regulatory framework, and implementation challenges in 2025.

A curated guide to the most reliable and comprehensive pharmaceutical industry news sources, specifically tailored for IT professionals working in pharma and life sciences.

An in-depth analysis of how artificial intelligence is transforming clinical data management across US healthcare, from EHR documentation to clinical trials and real-world evidence.

A technical deep-dive into Veeva's Closed Loop Marketing (CLM) platform, exploring implementation, integration, and optimization for pharmaceutical marketing teams.

A comprehensive guide to integrating Randomization and Trial Supply Management (RTSM) systems with Electronic Data Capture (EDC) platforms, covering benefits, challenges, and vendor solutions.

An in-depth analysis of top Clinical Research Management Systems in the United States, comparing features, benefits, and implementation strategies for pharmaceutical companies.

Discover how Veeva RTSM's real-time analytics transform clinical trials by optimizing patient randomization, supply management, and operational efficiency with data-driven insights.

Explore how MCP is revolutionizing data integration and AI applications in pharmaceutical research, clinical trials, and healthcare systems for enhanced compliance.

A comprehensive guide to regulatory requirements and best practices for implementing compliant Randomization and Trial Supply Management (RTSM) systems in clinical trials, covering FDA, EMA, and global standards.

A comprehensive guide to how Amazon Web Services (AWS) is transforming pharmaceutical operations from drug discovery to manufacturing, with real-world case studies from Pfizer, Moderna, Merck, and more.

Explore real-world case studies of how pharmaceutical companies are leveraging big data, AI, and cloud computing across the drug lifecycle - from discovery to marketing - with measurable outcomes and lessons learned.

A comprehensive guide to electronic patient record systems in pharmaceutical research and clinical trials, exploring their benefits, implementation challenges, and regulatory considerations.

A comprehensive exploration of generative AI proof of concepts in pharmaceutical research, examining real-world applications, implementation strategies, and measurable outcomes across the drug development pipeline.

A comprehensive analysis of how Google Cloud Platform (GCP) is revolutionizing pharmaceutical operations, from AI-powered drug discovery to clinical trial management and regulatory compliance.

An in-depth analysis of IBM's contributions to pharmaceutical innovation, from AI-powered drug discovery and hybrid cloud infrastructure to regulatory compliance and security solutions.

A comprehensive analysis of the life sciences job market in 2025, exploring emerging roles, skill requirements, salary trends, and career opportunities across pharmaceutical, biotechnology, and medical device sectors.

An in-depth exploration of how pharmaceutical companies leverage Microsoft Azure's cloud platform for drug discovery, clinical trials, manufacturing, and regulatory compliance, with real-world case studies and implementation strategies.

An in-depth guide to designing and implementing modern datacenter infrastructure for pharmaceutical companies, focusing on scalability, security, regulatory compliance, and integration with cloud services for drug development and manufacturing.

A comprehensive guide to implementing and optimizing NetSuite ERP for pharmaceutical companies, covering supply chain management, manufacturing operations, quality control, regulatory compliance, and integration with specialized pharma systems.

A comprehensive analysis of how pharmaceutical and biotech companies are leveraging NVIDIA's latest H100 and Blackwell GPUs to accelerate drug discovery, protein structure prediction, and AI-driven research, with detailed case studies from leading companies.

Comprehensive guide on RTSM best practices for Phase 3 trials, covering randomization strategies, global supply chain management, regulatory considerations (FDA), system integration, risk mitigation, and future trends.

Comprehensive analysis of big data technologies used in pharmaceutical industry, including Hadoop, Spark, cloud data warehouses, NoSQL databases, and specialized genomics platforms, with detailed comparisons and implementation examples.

Comprehensive guide on implementing CAPA dashboards in pharmaceutical quality management, covering data requirements, key metrics, dashboard design principles, and implementation in Power BI, Tableau, and Google Data Studio.

Comprehensive overview of how computer vision technologies are revolutionizing pharmaceutical quality control processes, from tablet inspection to packaging verification, with real-world implementation examples and ROI analysis.

Comprehensive overview of Oracle's role in the pharmaceutical industry, covering their Health Sciences solutions, cloud infrastructure, compliance features, and case studies of successful implementations at major pharma companies.

Practical guide for Market Access teams and field representatives on handling common payer objections in oncology, including strategies for verbal and written communications, real-world examples, and response frameworks.

A comprehensive analysis of how pharmaceutical companies leverage SAP's enterprise solutions for drug development, clinical trials, manufacturing, supply chain management, and regulatory compliance, with detailed case studies from leading pharma companies.

A comprehensive analysis of leading medical technology companies worldwide that are at the forefront of AI adoption, examining their innovative applications in medical imaging, diagnostics, robotic surgery, patient monitoring, and personalized medicine, with detailed profiles of each company's AI technologies and market impact.

A comprehensive guide to building effective performance dashboards for small hospitals, covering key metrics, data integration, visualization tools, and practical implementation strategies to improve decision-making and operational efficiency.

An in-depth analysis of the leading AI consulting firms serving U.S. pharmaceutical companies, highlighting their focus areas, technologies, and recent activities in drug discovery, compliance, efficiency, and commercial applications.

A comprehensive guide for pharmaceutical marketing teams to plan, build, and optimize a custom HCP engagement portal to boost physician engagement through educational content, interactive tools, and personalized experiences.

A comprehensive guide to Key Opinion Leader (KOL) tiering in pharmaceutical marketing, covering definition, strategic importance, tiering criteria, engagement strategies, and compliance considerations for U.S. pharma companies.

A detailed exploration of Non-Personal Promotion strategies in pharmaceutical marketing, covering best practices, compliance requirements, and tools for effective HCP engagement without face-to-face interaction.

A comprehensive step-by-step guide for pharmaceutical marketing teams to plan, develop, and launch effective patient portals that improve engagement, adherence, and outcomes while ensuring regulatory compliance.

A comprehensive list of 50+ events and conferences in 2025 that are relevant to Veeva professionals, including Veeva's own conferences, pharma IT events, and industry gatherings, all sorted by relevance score.

A comprehensive analysis of Workday's growing adoption in the pharmaceutical and life sciences sector, examining market trends, key drivers, notable industry users, competitive positioning against SAP, Oracle, and ADP, and future outlook for enterprise cloud solutions in life sciences.

An in-depth exploration of how data science is revolutionizing the life sciences industry, from drug discovery to clinical trials, with real-world applications and case studies.

Learn how to identify, segment, and engage Healthcare Professionals (HCPs) and Key Opinion Leaders (KOLs) effectively for pharmaceutical commercialization, including methodologies, data sources, tools, and compliance considerations.

A comprehensive guide to compliant pharmaceutical sales practices, covering FDA regulations, PhRMA Code requirements, and best practices for engaging healthcare professionals while avoiding common compliance pitfalls.

A comprehensive guide to how pharmaceutical companies identify, segment, and engage healthcare professionals (HCPs) for sales outreach, covering databases, targeting strategies, digital tools, and compliance considerations.

A comprehensive overview of the most influential open-source software tools transforming pharmaceutical research, development, and manufacturing, from cheminformatics to clinical data management and regulatory compliance.

A comprehensive guide to the leading commercial analytics software platforms for pharmaceutical companies, covering sales forecasting, field force effectiveness, market access analysis, real-world evidence integration, customer segmentation, and omnichannel marketing optimization.

A comprehensive guide to Veeva Approved Email, exploring how pharmaceutical companies use this compliant communication tool to engage healthcare providers while adhering to FDA regulations and industry compliance standards.

A detailed technical guide to all APIs available across the Veeva ecosystem, including REST, SOAP, Bulk APIs, and SDKs for Vault, CRM, Network, and OpenData, with authentication, versioning, and integration scenarios.

A comprehensive breakdown of Veeva Systems' pricing models, licensing structures, and cost considerations for life sciences companies evaluating Veeva Vault, CRM, and other products.

A comprehensive analysis of Veeva Systems as a long-term investment, examining its market position in life sciences software, financial performance, growth prospects, competitive landscape, and valuation metrics for 2025 and beyond.

A comprehensive training manual for pharmaceutical sales representatives on using Veeva Vault CRM, covering account management, call logging, CLM presentations, sample distribution, and data synchronization on iPad and desktop.

A comprehensive analysis of cloud computing adoption versus traditional on-premises infrastructure in the pharmaceutical industry, including current adoption rates, historical trends, future forecasts, and key drivers of cloud migration.

A comprehensive comparison of IQVIA's Orchestrated Customer Engagement (OCE) and Veeva CRM platforms, analyzing features, integrations, usability, support, compliance, security, mobile capabilities, scalability, and user feedback for pharmaceutical organizations.

A comprehensive guide for technical administrators on configuring user-level security in Veeva Vault, covering security profiles, permission sets, roles, and best practices for compliance.

A comprehensive guide to modern data warehousing solutions for life sciences organizations, covering cloud vs. on-premise strategies, technology stacks, compliance requirements, and scalable approaches for organizations of all sizes.

Practical strategies to boost Veeva CRM adoption among sales reps and MSLs, addressing common challenges like poor user experience, lack of training, and resistance to change.

A comprehensive overview of Customer Relationship Management (CRM) platforms tailored for biotech companies, comparing various solutions and their features for compliance, sales, and customer relationship management in the life sciences sector.

A comprehensive guide to modern healthcare professional (HCP) engagement solutions for pharmaceutical companies, covering omnichannel strategies, compliance requirements, technology platforms, and best practices for tracking and measuring engagement effectiveness.

A comprehensive guide for pharmaceutical marketers on planning and executing market access pull-through campaigns using Veeva CRM and Vault, from stakeholder targeting to field execution.

A comprehensive analysis of how pharmaceutical marketing differs from traditional industries, covering regulatory frameworks, key players, allowed practices, and compliance requirements in the U.S. healthcare market.

An in-depth analysis of the five most digitally innovative pharmaceutical companies in Europe, examining their AI initiatives, digital transformation strategies, and how they're leveraging technology to accelerate drug development and improve patient outcomes.

A comprehensive comparison of CRM requirements between pharmaceutical companies and other life sciences organizations, examining key differences in sales, marketing, compliance, and patient engagement approaches.

A comprehensive comparison of three approaches to adapting large language models for pharmaceutical applications: fine-tuning, distillation, and prompt engineering, with technical details and real-world examples.

A comprehensive analysis of Veeva's decision to end its partnership with Salesforce, exploring the strategic implications, timeline of events, and impact on life sciences companies facing this major industry transition.

A comprehensive overview of the worldwide ecosystem of Veeva consulting partners, highlighting regional specialists and global players in the life sciences sector.
An in-depth analysis comparing Veeva CRM with other CRM solutions in the life sciences industry, examining features, compliance, and industry-specific capabilities.

In-depth analysis of Veeva Systems' transformation from 2021 to 2025, examining strategic shifts, product innovations, and market leadership in life sciences cloud solutions.

An in-depth look at Veeva Systems' office locations worldwide, including architecture, workplace culture, team distributions, recent expansions, and future plans for global presence.

A detailed comparison of Veeva Align and Align+, exploring how these complementary tools transform territory planning and management for sales, medical, and marketing teams in life sciences.

A detailed analysis of Veeva CTMS features and capabilities, exploring how it streamlines clinical trial operations and improves study execution.

A comprehensive guide to Veeva Compass Suite's features and benefits, exploring how it transforms commercial operations through comprehensive claims data for sales, marketing, and analytics teams.

A comprehensive overview of Veeva Engage, exploring how this cloud-based platform enables pharmaceutical and life sciences companies to interact with healthcare professionals (HCPs) through compliant digital channels, including virtual meetings, messaging, and content sharing.

A comprehensive technical analysis of Veeva Vault eTMF, exploring its architecture, key features, and integration capabilities.

A comprehensive overview of Veeva FormTrak, exploring how this MMIT-powered solution enables pharmaceutical field teams to deliver timely, relevant access messaging to healthcare providers through Veeva CRM.

A comprehensive technical guide for developers on creating custom dashboards in Veeva CRM using MyInsights, covering architecture, data access, and best practices.

A comprehensive technical overview of Veeva Nitro, exploring how this cloud-based data warehouse platform revolutionizes commercial data management and analytics in the pharmaceutical industry through pre-built connectors, industry-specific data models, and integrated analytics capabilities.

A comprehensive overview of Veeva OpenData, exploring how this global customer reference data solution provides pharmaceutical companies with accurate, up-to-date information on healthcare professionals (HCPs) and healthcare organizations (HCOs) to power their commercial operations.

A comprehensive guide to Veeva PromoMats features and capabilities, exploring how it streamlines promotional content management and ensures regulatory compliance in life sciences.

A detailed technical overview of Veeva's Randomization and Trial Supply Management (RTSM) system, covering architecture, features, integration capabilities, and regulatory compliance for clinical trials.

A comprehensive guide to integrating Veeva CRM with SAP Concur, exploring how this integration transforms enterprise workflows by streamlining expense management and ensuring compliance in life sciences.

A comprehensive technical guide to Veeva Vault Platform, covering architecture, development capabilities, integration options, and security features for software developers.