
Analyze FDA Project Elsa, a generative AI system that prioritizes facility inspections by detecting risk patterns in adverse events and regulatory data.

Analyze FDA Project Elsa, a generative AI system that prioritizes facility inspections by detecting risk patterns in adverse events and regulatory data.

Analyze the FDA Quality Management System Regulation (QMSR) effective Feb 2, 2026. Learn how ISO 13485:2016 incorporation impacts medical device compliance.

Analyze FDA QMSR requirements for DHF remediation. Learn how to transition legacy Design History Files to the ISO 13485 Medical Device File framework.

Analyze the readiness decay curve and why mock inspections fail. Learn how skill atrophy, CAPA drift, and cognitive biases erode regulatory compliance.

Analyze the pharmaceutical AI skills gap and workforce upskilling strategies. Review regulatory impacts, training models, and ROI metrics for R&D teams.

Analysis of FDA digital health guidance covering SaMD, AI, and cybersecurity. Understand 2026 updates, risk categorization, and regulatory compliance pathways.

Explore how AI automates CAPA and deviation workflows in Veeva Vault QMS. Covers NLP triage, ML root cause analysis, and regulatory compliance efficiency.

Configure CAPA management in Veeva Vault QMS with this technical guide. Covers data models, 21 CFR Part 820 compliance, and workflow implementation steps.

Compare Oracle Argus, ArisGlobal LifeSphere, and Veeva Vault Safety. This guide covers PV database features, compliance, and cloud architecture differences.

Examine how Veeva Vault QMS supports ISO 13485 compliance for medical devices. Learn to map digital QMS features to regulatory standards for audit readiness.

Analyze Veeva implementation costs for Series B/C biotechs. Review licensing fees, professional services, and TCO models for Vault and CRM deployments.

Examine the risks of DIY quality systems in biotech. Learn about FDA Part 11 compliance, data integrity, and when to transition from Excel to eQMS software.

Explore the four layers of a modern biotech software stack—infrastructure, data, apps, and analytics—essential for scaling R&D before Series C funding.

Explore the FDA PCCP framework for medical devices. Understand Section 515C requirements for pre-approving AI/ML software changes without new submissions.

Understand FDA SaMD classification for AI/ML devices. Review risk levels (Class I-III), 510(k) pathways, and regulatory guidelines for medical software.

Understand EU MDR and AI Act compliance for AI medical devices. Explains classification, conformity assessment, and technical documentation requirements.

Analyze the life sciences software market projected to reach $45B by 2026. Examine AI trends, key segments, and five structural gaps hindering growth.

Analyze AI integration in pharma IT architecture, from R&D to supply chain. Review key data on MLOps, clinical trial efficiency, and FDA guidance.

Learn how AI automates adverse event detection in pharmacovigilance. This guide covers GVP compliance, NLP methods, and validation standards for safety data.

Learn what Approved WeChat on WeCom is and how it enables compliant pharmaceutical engagement with HCPs in China. Explore its features, CRM integration, and rol

Learn to implement an audit-ready ISO 27001 ISMS for life sciences. This guide covers integrating cybersecurity with GxP, QMS, and FDA regulatory compliance.

Understand analytical method validation with this deep dive into ICH Q2(R2). Explore validation parameters, documentation, and the new QbD lifecycle approach wi

Learn the integrated workflow for manufacturing deviations, CAPA, and change control in GMP. This guide covers regulatory requirements and common inspection pit

Explore how AI Contract Lifecycle Management (CLM) software streamlines biotech contract workflows, ensures regulatory compliance, and accelerates clinical tria

Compare the IEC 62304 standard vs. FDA CSA guidance for medical software. Learn key differences in scope, risk classification, validation, and documentation.

Learn how to build SAP dashboards for pharma and biotech. This guide covers data architecture, key KPIs, SAP tools like Analytics Cloud, and regulatory complian

A comprehensive analysis of how data quality and data culture are foundational to AI success in pharmaceutical and life sciences organizations, covering assessment frameworks, governance models, regulatory compliance, and practical implementation roadmaps.

Learn how to use generative AI for SOP drafting in GxP life sciences. This guide covers balancing automation benefits with regulatory compliance, validation, an

Learn why spreadsheets fail for life sciences contract management. This guide explores how AI-powered CLM improves efficiency, compliance, and clinical trial cy

Explore how GenAI helps manage pharmaceutical labeling. Learn to draft local label deviations from a CCDS and understand AI's role in regulatory compliance.

Explore the career path from a Regulatory Affairs Generalist to a specialized Labeling Strategist. Learn the key skills, role differences, and industry trends.

Learn how Veeva RIM automates end-to-end pharmaceutical labeling. This guide explains 5 core workflows for label change management and regulatory compliance.

Learn how Veeva PromoMats' Auto-Update Anchors feature automates marketing claims substantiation to ensure regulatory compliance and reduce manual MLR review ef

This comprehensive 2025 guide details e-labeling (e-IFU) regulations worldwide. See our country-by-country analysis of QR code acceptance for medical devices &

A comprehensive guide to transitioning from XML-based SPL to HL7 FHIR for e-labeling. Learn the key benefits, regulatory requirements, and steps for team readin

A detailed guide to the 13 principles of Good Clinical Practice (GCP). Understand the ICH E6(R2) standards for protecting subjects and ensuring data integrity.

A guide to the clinical trial protocol. Learn its core components, importance in research, and how to manage complexity and amendments for successful trials.

Learn about key clinical trial acronyms. This guide explains the definitions, history, and roles of GCP, ICH, IRB, EDC, and eTMF in clinical research.

A complete guide to clinical trial site close-out. Our checklist covers data integrity, IP accountability, archiving, and regulatory compliance per ICH-GCP & FD

Learn about 21 CFR Part 58, the FDA's Good Laboratory Practice (GLP) regulations for nonclinical studies. This guide covers its scope, history, and key subparts

A technical guide to creating a GLP-compliant audit trail on macOS. Learn to meet data integrity rules (21 CFR Part 11) using APFS, FSEvents & Endpoint Security

Explore the FDA Pre-license Inspection (PLI) process for biologics. This guide explains the PLI's purpose, scope, and cGMP requirements for BLA approval.

An educational guide to leading pharmacovigilance service providers. Learn about the PV outsourcing market, regulatory drivers, and compare top drug safety CROs

A comprehensive guide to MLR review software for pharma and life sciences. Compare the top 10 platforms and learn how to ensure marketing content compliance.

A comprehensive 2025 guide to ICH Q7, Q8, & Q9. Learn the core principles of GMP for APIs (Q7), Quality by Design/QbD (Q8), and Quality Risk Management/QRM (Q9)

Explore how AI impacts Good Documentation Practice (GDocP) and ALCOA+ principles in life sciences. Learn about efficiency gains, data integrity risks, and new r

Learn about ISO 13485:2016, the quality management system (QMS) standard for medical devices. This guide explains its requirements, purpose, and role in complia

Is ISO 9001 still used in biotech? This guide analyzes how the QMS standard supports regulatory compliance, complements GMP, and improves quality in pharma & li

Learn how to implement Veeva Vault PromoMats for life sciences. This guide provides a step-by-step playbook for setting up compliant MLR review workflows.

A detailed 2025 analysis of the GxP managed services market for pharma & life sciences. Learn about trends, regulatory drivers, and GxP compliance challenges.

Learn about Veeva Vault eTMF, the leading electronic trial master file solution. Updated for 2026 with AI Agents, TMF RM v4, ICH E6(R3) compliance, and 500+ customers

Learn the 9 ALCOA+ principles for GxP data integrity. Updated for 2026 with ICH E6(R3) finalization, EU GMP Chapter 4 ALCOA++ draft, and latest FDA enforcement trends

Learn why automating Veeva Vault metadata change detection is vital for life sciences compliance. Updated for 2026 with Direct Data API, Action Triggers, and Veeva AI Agents.

Learn what an electronic Investigator Site File (eISF) is and its role in clinical trials. Updated for 2026 with ICH E6(R3) finalization, latest adoption data, and eISF vs. eTMF comparison

Learn about the TMF Reference Model (now TMF Standard Model under CDISC), the industry-standard taxonomy for organizing Trial Master File documents in clinical trials. Covers TMF SM v1, ICH E6(R3) alignment, and AI-powered eTMF automation.

Updated 2026 guide to Veeva SiteVault, the free eISF solution for clinical research sites. Covers CTMS launch, eSource, AI Agents, compliance (21 CFR Part 11), and impact on site operations

Prepare for your life sciences role with our updated 2026 guide to top Veeva Vault interview questions. Covers Vault CRM migration, AI Agents, platform features, and compliance topics

An in-depth analysis of the 2025 Computer System Validation (CSV) job market. Learn about validation specialist salaries, key skills, and career trends.

An in-depth guide to IT system assessment in pharma M&A due diligence. Covers infrastructure, data, cybersecurity, AI governance, NIS2/CRA compliance, and FDA cybersecurity guidance for 2025-2026 deals.

A comprehensive guide to lab equipment qualification (IQ/OQ/PQ). Learn the process, regulatory standards including FDA's finalized CSA guidance, and how AI-powered automation streamlines validation and compliance.

An in-depth analysis of the ICH E6(R3) Good Clinical Practice guidelines for 2025. Explore key changes in Quality by Design, data governance, and decentralized

An in-depth guide to ICH Q10 Pharmaceutical Quality Systems (PQS), updated for 2026 with ICH Q9(R1), Q12-Q14 developments, FDA QMM program updates, and AI-driven quality management trends.

Learn best practices for writing an effective FDA 483 response. Updated with FY2024-2025 enforcement data and FDA's AI-driven inspection targeting. Covers CAPAs, timelines, and strategies to avoid Warning Letters.

Examines the 2025 AI regulatory frameworks for biopharma in the U.S., EU, U.K., and Canada. Details compliance obligations for GenAI, GxP, and SaMD.

Updated guide to GAMP 5 guidelines for validating computerized systems, covering the 2025 ISPE GAMP AI Guide, FDA CSA final guidance, EU Annex 11/22 drafts, and risk-based lifecycle approach.

An updated analysis of leading Regulatory Information Management (RIM) systems for 2026, covering Veeva Vault RIM, IQVIA SmartSolve RIM, ArisGlobal LifeSphere, Ennov, MasterControl, EXTEDO, and others, with the latest on eCTD 4.0, IDMP compliance, and AI-powered automation.

Updated for 2026, this article provides a technical overview of pharmacovigilance software, detailing core functions, regulatory compliance, agentic AI developments, and a comparison of leading platforms including Oracle Argus, ArisGlobal LifeSphere, Veeva Vault Safety, and more.

Learn what Policy as Code (PaC) is and why it is used in healthcare to automate enforcement of security, privacy, and compliance regulations, including the latest HIPAA Security Rule updates and OPA 1.0 developments.

A comprehensive guide to the IEC 62304 standard for medical device software, updated for 2026. Covers life cycle processes, safety classification, Edition 2.0 draft changes, FDA QMSR alignment, IEC 81001-5-1 cybersecurity, and regulatory compliance across FDA and EU MDR.

An analysis of top ERP systems for the pharmaceutical sector, evaluated on regulatory compliance (21 CFR Part 11), batch traceability, and serialization. Updated for 2025–2026 with DSCSA enforcement timelines, AI/Copilot capabilities, and latest platform releases.

This article explains the core functions of LIMS for sample management, data integrity, and regulatory compliance, and provides a comparative analysis of top systems.

An explanation of Clinical Data Management (CDM) and its function in research. Learn how CDM ensures high-quality, reliable data for clinical trials.

A comprehensive guide to pharmaceutical KPIs for quality, compliance, and operational excellence. Covers metrics for R&D, clinical trials, manufacturing, supply chain, and pharmacovigilance, updated with 2025-2026 regulatory developments including FDA QMM, ICH E6(R3), and ISO 9001:2026.

Updated for 2026, this article examines the unique financial challenges facing regional hospitals — including 46% operating at negative margins — and details the accounting practices, software solutions, and AI-driven automation essential for maintaining stability and compliance.

A comprehensive overview of AI in pharmacovigilance (updated Feb 2026), covering agentic AI, GenAI-driven case processing, signal detection, CIOMS WG XIV framework, FDA/EMA joint principles, EU AI Act implications, and the latest industry platforms.

Learn how WORM (Write Once, Read Many) storage ensures data immutability and integrity for electronic records in the highly regulated biotechnology industry. Updated for 2026 with the latest on EU GMP Annex 11 revisions, SEC 17a-4 audit-trail alternatives, cloud WORM advances, and ransomware defense strategies.

An educational guide to executing compliant pharmaceutical remarketing. Explores platform policies on Google, Meta, LinkedIn, and Reddit, plus data privacy. Updated for 2025-2026 with Meta's health-data restrictions, FDA enforcement escalation, Google policy changes, and HIPAA tracking updates.

Examines RegTech adoption challenges for emerging biotechs, focusing on cost, scalability, and AI-driven compliance. Covers flexible licensing, usage-based pricing, deferred-value models, and the latest platform innovations from Veeva Vault Basics and beyond.

This article examines strategies for integrating content management systems like Box and Veeva Vault to overcome information silos in regulated environments.

An analysis of how CRM systems support medical device firms in managing HCP relationships, sales pipelines, and maintaining strict regulatory compliance.

This report details how pharmaceutical companies use mobile apps for patient support, medication adherence, and data collection, including the 2023-2024 digital therapeutics shakeout, FDA regulatory updates through 2026, and emerging AI-powered engagement strategies.

Explore how ERP systems integrate biotech R&D, manufacturing, and finance, ensuring GxP compliance and traceability. Covers major and niche vendor solutions.

Explore key software needs, technology stacks, and specific tools like AI-driven drug design and cheminformatics in pharmaceutical software development.

This article explains pharmaceutical serialization software, its critical role in securing the drug supply chain, and adherence to regulations like DSCSA and FMD. Updated with 2025-2026 enforcement deadlines and vendor developments.

Evaluate top 10 accounting/ERP solutions for pharmaceutical companies. Learn about features like regulatory compliance, batch tracking, and quality control systems.

Learn how Power BI consultants apply business intelligence to pharmaceutical data, addressing regulatory needs, clinical trial insights, and RWE analysis.

This article details AI's role in biotech sample management, covering traditional workflows, challenges, AI innovations, regulatory issues, and future outlook.

Learn about the 2025 job market for Computer System Validation (CSV) professionals. Understand CSV's critical role in regulated industries for data integrity and patient safety.

Learn about regulatory compliance software in pharmaceuticals, covering GMP, GLP, GCP, FDA 21 CFR Part 11, and EU Annex 11 standards for data integrity.

Compare GAMP 4 and GAMP 5. Learn key differences in their approaches to system validation, risk management, system classification, and integration of modern technologies. Updated with 2025 FDA CSA guidance and ISPE GAMP AI Guide.

Explore the distinct roles and benefits of Learning Management Systems (LMS), Digital Adoption Platforms (DAP), and Knowledge Management Systems (KMS) in life sciences.

Compares NetSuite and SAP ERP systems' technical capabilities and regulatory compliance features for pharmaceutical and life sciences companies.

Explore Good Manufacturing Practice (GMP) in pharma. Learn its principles, global regulations, implementation, and compliance challenges for quality assurance.

This guide explains GAMP 5, its principles, and lifecycle for computerized system validation in pharma, covering regulatory compliance and real-world applications. Updated for 2025-2026 with FDA CSA guidance, EU Annex 11 revision, and ISPE AI Guide.

Learn to build a pharmaceutical manufacturing plant from concept through commissioning. This guide covers planning, regulatory compliance, design, equipment, quality, validation, and approval.
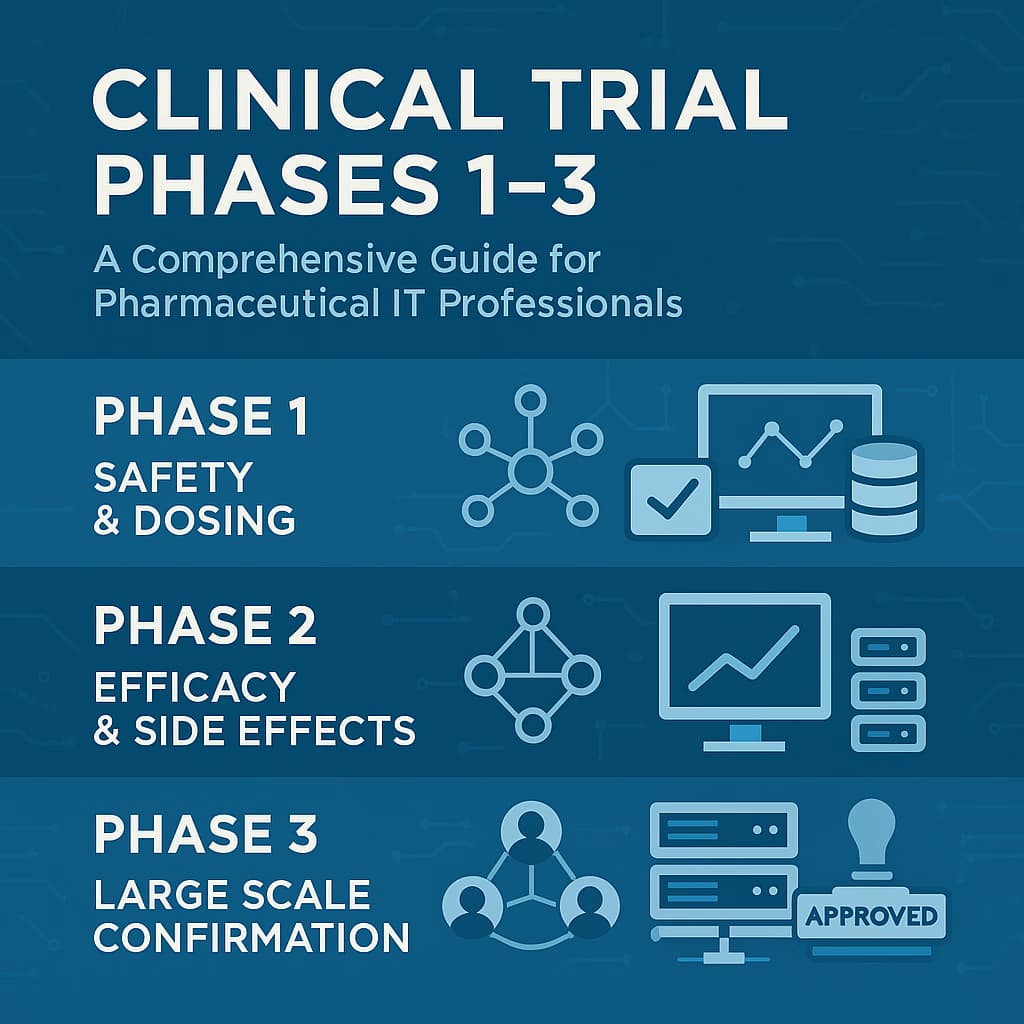
A practical guide to clinical trial phases 1–3 for pharma IT: objectives, compliance, costs, and IT infrastructure for modern drug development.

An analysis of how artificial intelligence is transforming regulatory affairs in pharmaceuticals, from submission preparation to compliance monitoring and regulatory intelligence.

Step-by-step guide to all Veeva Vault login methods: username/password, SSO, mobile app, and external/partner access. Updated for 2026 with new MFA, session controls, and 26R1 features. Includes troubleshooting, FAQs, and application-specific notes.

A comprehensive analysis of ChatGPT integration in life sciences, examining implementation strategies, regulatory compliance, and real-world applications across pharmaceutical research and development.

A comprehensive analysis of the remote patient monitoring landscape in the US healthcare system, examining technological advances, regulatory framework, and implementation challenges in 2025.

An in-depth analysis of how artificial intelligence is transforming clinical data management across US healthcare, from EHR documentation to clinical trials and real-world evidence.

A comprehensive guide to integrating Randomization and Trial Supply Management (RTSM) systems with Electronic Data Capture (EDC) platforms, covering benefits, challenges, and vendor solutions.

An in-depth analysis of top Clinical Research Management Systems in the United States, comparing features, benefits, and implementation strategies for pharmaceutical companies.

A comprehensive guide to regulatory requirements and best practices for implementing compliant Randomization and Trial Supply Management (RTSM) systems in clinical trials, covering FDA, EMA, and global standards.

A comprehensive guide to how Amazon Web Services (AWS) is transforming pharmaceutical operations from drug discovery to manufacturing, with real-world case studies from Pfizer, Moderna, Merck, and more.

A comprehensive guide to electronic patient record systems in pharmaceutical research and clinical trials, exploring their benefits, implementation challenges, and regulatory considerations.

A comprehensive analysis of how Google Cloud Platform (GCP) is revolutionizing pharmaceutical operations, from AI-powered drug discovery to clinical trial management and regulatory compliance.

An in-depth analysis of IBM's contributions to pharmaceutical innovation, from AI-powered drug discovery and hybrid cloud infrastructure to regulatory compliance and security solutions.

An in-depth exploration of how pharmaceutical companies leverage Microsoft Azure's cloud platform for drug discovery, clinical trials, manufacturing, and regulatory compliance, with real-world case studies and implementation strategies.

An in-depth guide to designing and implementing modern datacenter infrastructure for pharmaceutical companies, focusing on scalability, security, regulatory compliance, and integration with cloud services for drug development and manufacturing.

A comprehensive guide to implementing and optimizing NetSuite ERP for pharmaceutical companies, covering supply chain management, manufacturing operations, quality control, regulatory compliance, and integration with specialized pharma systems.

Comprehensive guide on implementing CAPA dashboards in pharmaceutical quality management, covering data requirements, key metrics, dashboard design principles, and implementation in Power BI, Tableau, and Google Data Studio.

Comprehensive overview of how computer vision technologies are revolutionizing pharmaceutical quality control processes, from tablet inspection to packaging verification, with real-world implementation examples and ROI analysis.

Comprehensive overview of Oracle's role in the pharmaceutical industry, covering their Health Sciences solutions, cloud infrastructure, compliance features, and case studies of successful implementations at major pharma companies.

A comprehensive analysis of how pharmaceutical companies leverage SAP's enterprise solutions for drug development, clinical trials, manufacturing, supply chain management, and regulatory compliance, with detailed case studies from leading pharma companies.

An in-depth analysis of the leading AI consulting firms serving U.S. pharmaceutical companies, highlighting their focus areas, technologies, and recent activities in drug discovery, compliance, efficiency, and commercial applications.

A comprehensive overview of the most influential open-source software tools transforming pharmaceutical research, development, and manufacturing, from cheminformatics to clinical data management and regulatory compliance.

A comprehensive analysis of cloud computing adoption versus traditional on-premises infrastructure in the pharmaceutical industry, including current adoption rates, historical trends, future forecasts, and key drivers of cloud migration.

A comprehensive analysis of how pharmaceutical marketing differs from traditional industries, covering regulatory frameworks, key players, allowed practices, and compliance requirements in the U.S. healthcare market.

A comprehensive guide to Veeva PromoMats features and capabilities, exploring how it streamlines promotional content management and ensures regulatory compliance in life sciences.
© 2026 IntuitionLabs. All rights reserved.