
Learn to validate AI vendor claims in pharma. This due diligence checklist covers GxP compliance, data security, and model performance verification methods.

Learn to validate AI vendor claims in pharma. This due diligence checklist covers GxP compliance, data security, and model performance verification methods.

Examine the risks of DIY quality systems in biotech. Learn about FDA Part 11 compliance, data integrity, and when to transition from Excel to eQMS software.

An overview of regulatory submission software for the life sciences. Learn how RIM systems & eCTD publishing tools streamline compliant filings to the FDA & EMA

Explore top Veeva Vault RIM alternatives for life sciences. This guide compares leading RIM systems from IQVIA, ArisGlobal, and more on features, cost, and ROI.

Learn how Regulatory Information Management (RIM) systems help biotech firms manage compliance. This guide details core capabilities, submission planning, and t

Learn what Approved WeChat on WeCom is and how it enables compliant pharmaceutical engagement with HCPs in China. Explore its features, CRM integration, and rol

Learn the complete syntax of Veeva Vault Query Language (VQL). This guide covers SELECT, FROM, WHERE, and FIND clauses, API usage, and practical examples.

Get a complete 50-task checklist for the Veeva CRM to Vault CRM migration. This guide covers data quality, integrations, and the 2025-2030 transition from Sales

An in-depth analysis of the build vs. buy decision for a quality management system (QMS) in biotech startups. Explore costs, timelines, and compliance factors.

Learn to apply the GAMP 5 risk-based approach for AI/ML validation in GxP environments. This guide covers data integrity, model drift, and regulatory compliance

A comprehensive analysis of how data quality and data culture are foundational to AI success in pharmaceutical and life sciences organizations, covering assessment frameworks, governance models, regulatory compliance, and practical implementation roadmaps.
Complete guide to Veeva X-Pages development services for Vault CRM. Covers X-Pages Studio vs custom development, timelines, costs, and how to select a development partner.

Analyze ServiceNow use cases in healthcare and life sciences. This guide covers ITSM for clinical workflows, patient experience, and compliance (HIPAA, GxP) wit

Learn how to use generative AI for SOP drafting in GxP life sciences. This guide covers balancing automation benefits with regulatory compliance, validation, an

This comprehensive guide explains the Veeva Direct Data API. Learn its features, use cases for bulk data extraction, and how it enables analytics and AI on Veev
Explore the Boston-Cambridge biotech ecosystem. This guide covers top companies like Biogen & Vertex, venture funding, and Massachusetts' drug development pipel

Explore San Diego's biotech hub, a top 3 U.S. cluster. This guide covers key companies like Illumina, economic data, and the history of its life sciences indust

A technical review of Gemini Nano Banana Pro, Google’s AI image model. Learn its key specs, 1M-token context, and specific applications in the life sciences ind

Explore how GenAI helps manage pharmaceutical labeling. Learn to draft local label deviations from a CCDS and understand AI's role in regulatory compliance.

Learn how Veeva RIM automates end-to-end pharmaceutical labeling. This guide explains 5 core workflows for label change management and regulatory compliance.

An analysis of Google's Gemini 3 AI for healthcare, pharma, and biotech. Learn about its multimodal reasoning, agentic features, and applications in drug discov

An educational review of top FDA compliance monitoring companies. Compare leading QMS software platforms and expert regulatory consulting firms for pharma & med

Is ISO 9001 still used in biotech? This guide analyzes how the QMS standard supports regulatory compliance, complements GMP, and improves quality in pharma & li

Learn about OCR and Document AI performance on real pharma documents. This report analyzes accuracy benchmarks for data extraction from clinical trials and SOPs

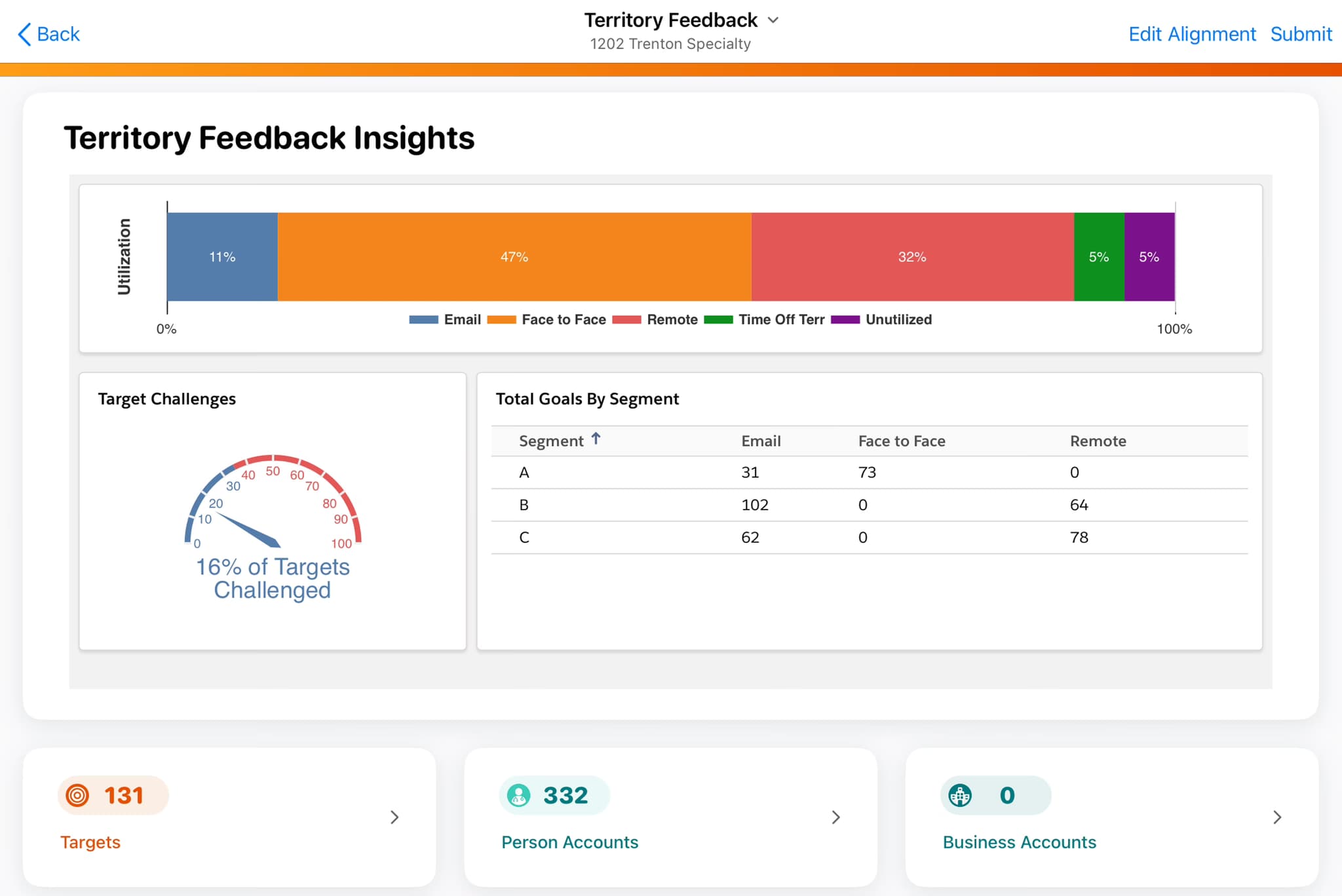
Learn the process for Veeva Align territory design in life sciences. This guide outlines a 4-week plan for data-driven alignment to boost sales productivity.

Learn how to implement Veeva Vault PromoMats for life sciences. This guide provides a step-by-step playbook for setting up compliant MLR review workflows.

A guide to Veeva OpenData onboarding for life sciences. Learn to identify and avoid common data quality pitfalls like duplicates and outdated HCP/HCO records.

A technical guide to Veeva and Salesforce data sync for life sciences. Learn about integration patterns, architecture, data mapping, and tools like MuleSoft & B

A technical guide to Veeva data integration for life sciences. Compare Veeva Nitro vs. Snowflake for your data warehouse, covering data models, pipelines, and c

An in-depth guide to Veeva Vault RIM. Understand the core modules like Submissions & Registrations and its role in modern Regulatory Information Management.

An in-depth guide to Veeva Vault. Learn how this cloud platform for life sciences manages regulated content, ensures GxP compliance, and unifies data management

Learn to build a compliant ETL pipeline from Veeva Vault to an Amazon S3 data lake. This guide covers data extraction APIs, architecture, AWS tools, and GxP nee

A detailed 2025 analysis of the GxP managed services market for pharma & life sciences. Learn about trends, regulatory drivers, and GxP compliance challenges.

An educational analysis of AI biotech funding in 2025. Explore venture capital investment trends, funding data, and key deals in AI for drug discovery.

Comprehensive guide to Regulatory Information Management (RIM) systems and ISO IDMP standards, covering EMA PMS deadlines, eCTD v4.0 timelines, AI-powered RIM tools, and compliance strategies for life sciences organizations (updated March 2026).

Prepare for your life sciences role with our updated 2026 guide to top Veeva Vault interview questions. Covers Vault CRM migration, AI Agents, platform features, and compliance topics

Learn about Veeva Vault RIM, the leading regulatory information management platform. Updated for 2026, this guide covers features, AI Agents, architecture, market adoption, and ROI with

Updated 2026 framework for validating generative AI in GxP systems. Covers 21 CFR Part 11, EU Annex 22, ISPE GAMP AI Guide, FDA CSA guidance, and FDA-EMA joint AI principles

An in-depth analysis of 1,000+ medical device companies in the SF Bay Area, covering the medtech ecosystem, key players like Intuitive Surgical ($10B+ revenue), Penumbra ($14.5B acquisition), and CeriBell's IPO, plus VC funding and market trends through 2026.

An educational guide to HPC in life sciences. We review top lab IT specialists and solutions for genomics, drug discovery, and bioinformatics data analysis.

An analysis of how Docusign (formerly DocuSign) is used for electronic signatures in pharma and life sciences. Learn how it can meet FDA 21 CFR Part 11 compliance with its Life Sciences modules and IAM platform, updated for 2026.

Learn about Lorenz docuBridge, a key eCTD publishing software for biotech. Updated for 2026 with v25.2 features, eCTD v4.0 EU support, verifAI, and 2,000+ installations

Learn about Dassault Systèmes' QUMAS EDMS for life sciences. This guide covers its features, EDMS 2026 release, AI integration, and use cases for GxP and 21 CFR Part 11 compliance.

An in-depth analysis of Cognizant's RapidPro tool for Veeva Vault migration. Learn how this certified accelerator streamlines GxP data migration into the Veeva QualityDocs platform, with 2026 updates on Vault AI Agents, the Salesforce separation wave, and expanded partner ecosystem

A 2026-updated technical comparison of Databricks vs. Snowflake for life sciences. Explore the lakehouse and AI data cloud for genomics, clinical data, Mosaic AI, Cortex AI, and ML workloads.

Explore biotech salary trends across global regions, updated with 2024-2025 data. Compares pay in top hubs like the US, Switzerland, and Asia-Pacific with cost-of-living analysis and funding recovery trends.

Updated 2026 guide to Veeva Vault certification. Learn about the restructured training tracks, $300 exam fees, five certification paths, Veeva AI Agents, maintenance requirements, and career ROI

Explore Salesforce Health Cloud and Agentforce Life Sciences for biopharma. Updated for 2026 with Life Sciences Cloud GA, Agentforce AI, and new customer wins from Novartis and AstraZeneca.

A data-driven analysis of the US biotech job market in 2025. Learn about employment trends, the skills gap, top geographic hubs, and the industry's future outlo

Updated for 2026, this article profiles five leading technology companies in healthcare AI—IntuitionLabs, AWS, Google Cloud, IBM, and Microsoft—examining their latest solutions for clinical workflows, AI agents, and patient engagement.

Learn about Apache Airflow's core architecture (including Airflow 3.x features like DAG versioning, Task Execution API, and HITL workflows), and its application for building data workflows in life sciences.

Examines the 2025 AI regulatory frameworks for biopharma in the U.S., EU, U.K., and Canada. Details compliance obligations for GenAI, GxP, and SaMD.

An updated analysis of leading Regulatory Information Management (RIM) systems for 2026, covering Veeva Vault RIM, IQVIA SmartSolve RIM, ArisGlobal LifeSphere, Ennov, MasterControl, EXTEDO, and others, with the latest on eCTD 4.0, IDMP compliance, and AI-powered automation.

Examines Veeva CRM and Vault CRM adoption across pharma and life sciences as of 2026, covering the Salesforce separation, Vault CRM migration, AI agents, and the competitive dynamics with Salesforce Agentforce Life Sciences.

This article examines strategies for integrating content management systems like Box and Veeva Vault to overcome information silos in regulated environments.

An analysis of how CRM systems support medical device firms in managing HCP relationships, sales pipelines, and maintaining strict regulatory compliance.

Explore how ERP systems integrate biotech R&D, manufacturing, and finance, ensuring GxP compliance and traceability. Covers major and niche vendor solutions.

An analysis of AI applications for Veeva Systems in life sciences. Examines emerging consultancies, market trends, and use cases in regulatory and commercial ops.

Explore AWS cloud computing's role in life sciences for scalable data processing, HPC, and analytics. Learn how AWS facilitates innovation in biotech and pharma.

Explore how Oracle Cloud CX supports customer experience in life sciences, encompassing CRM, marketing, and service applications for compliant engagement with HCPs and patients.

Learn about key technical, regulatory, organizational, ethical, and financial barriers hindering AI adoption in life sciences, with emerging solutions including the latest FDA/EMA guidance and regulatory sandboxes.

This guide details a structured approach for Generative AI adoption in life sciences, covering strategy, governance, technology, training, and ethical considerations.

Learn how NetSuite ERP modules support U.S. pharmaceutical manufacturing labs, covering GMP, FDA (21 CFR Part 11) compliance, and integrations for quality and production. Updated for 2026 with NetSuite Next AI features and DSCSA compliance deadlines.

Compare GAMP 4 and GAMP 5. Learn key differences in their approaches to system validation, risk management, system classification, and integration of modern technologies. Updated with 2025 FDA CSA guidance and ISPE GAMP AI Guide.

Evaluate LMS platforms for life sciences. Understand key compliance requirements: FDA 21 CFR Part 11, GxP, electronic records, and audit trails for validated training.

Explore the distinct roles and benefits of Learning Management Systems (LMS), Digital Adoption Platforms (DAP), and Knowledge Management Systems (KMS) in life sciences.

Compares NetSuite and SAP ERP systems' technical capabilities and regulatory compliance features for pharmaceutical and life sciences companies.

How AI leaders like Amodei, Hassabis, and Huang are accelerating drug discovery, genomics, and life science breakthroughs—compressing decades into years. Updated January 2026 with AlphaFold 3, Claude for Life Sciences, NVIDIA Evo 2, and $3B+ pharma partnerships.
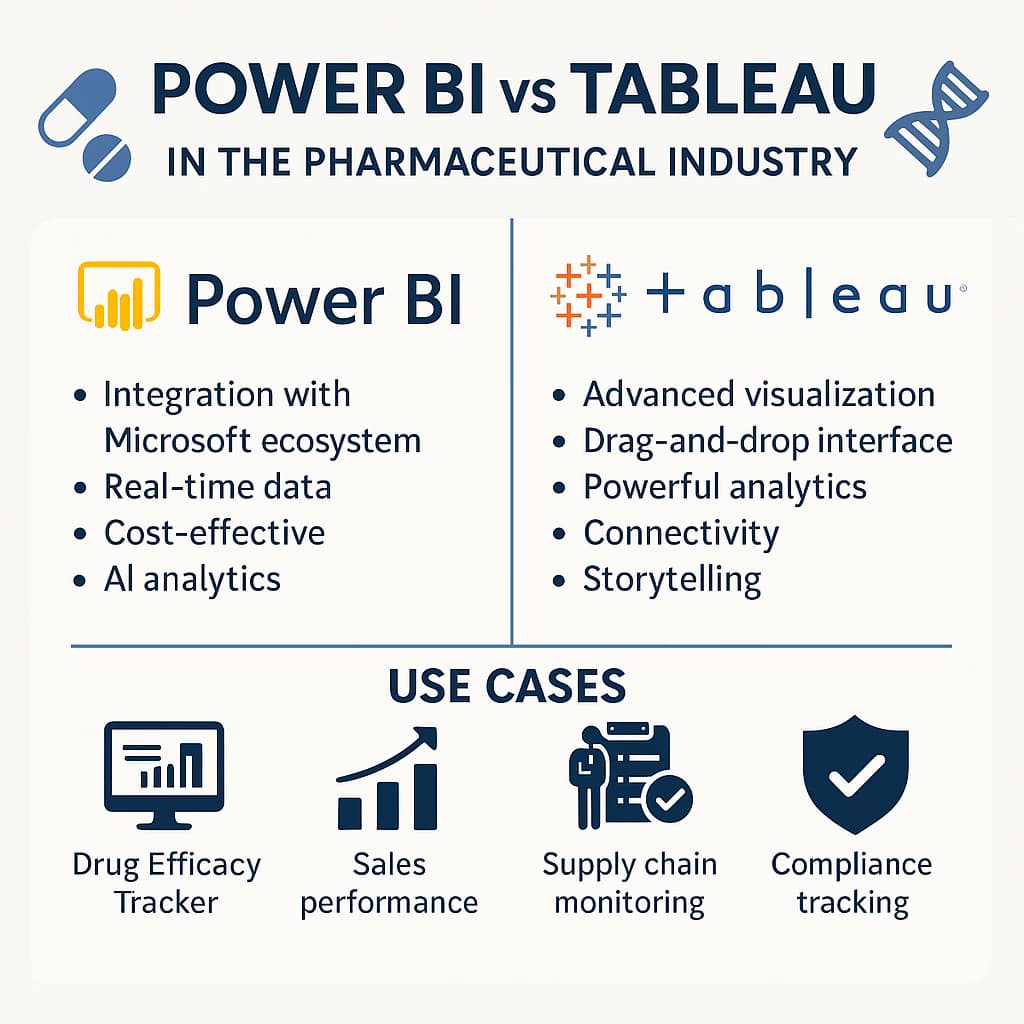
Comprehensive comparison of Power BI and Tableau for pharma: features, pricing, compliance, and use cases for IT and analytics teams.

A technical comparison of AI agents and AI workflows in pharmaceutical IT, with use cases, pros and cons, and adoption trends for U.S. pharma.

A detailed survey of large language model benchmarks in life sciences, covering biomedical NLP, drug discovery, and genomics, with industry use cases and top model performance.

A comprehensive review of software solutions for optimizing pharmaceutical field sales routes, comparing CRM, SFA, and mapping tools for efficiency, compliance, and analytics.

A comprehensive analysis of ChatGPT integration in life sciences, examining implementation strategies, regulatory compliance, and real-world applications across pharmaceutical research and development.

An in-depth analysis of the biotech ecosystem in the San Francisco Bay Area, examining key players, emerging startups, investment trends, and innovation clusters as of January 2026. Updated with latest data on 23andMe bankruptcy, major acquisitions, and 2025 layoffs.

A technical deep-dive into Veeva's Closed Loop Marketing (CLM) platform, exploring implementation, integration, and optimization for pharmaceutical marketing teams.

An in-depth analysis of top Clinical Research Management Systems in the United States, comparing features, benefits, and implementation strategies for pharmaceutical companies.

Explore how MCP is revolutionizing data integration and AI applications in pharmaceutical research, clinical trials, and healthcare systems for enhanced compliance.

A comprehensive analysis of the life sciences job market in 2025, exploring emerging roles, skill requirements, salary trends, and career opportunities across pharmaceutical, biotechnology, and medical device sectors.

Comprehensive guide on RTSM best practices for Phase 3 trials, covering randomization strategies, global supply chain management, regulatory considerations (FDA, ICH E6(R3)), system integration, risk mitigation, and future trends including AI-driven forecasting and decentralized trial support.

Comprehensive analysis of big data technologies used in pharmaceutical industry, including Hadoop, Spark 4.x, cloud data warehouses (Snowflake, Databricks), NoSQL databases, and specialized genomics platforms, with detailed comparisons and implementation examples. Updated for 2025-2026 with latest market data and technology developments.

Comprehensive guide on implementing CAPA dashboards in pharmaceutical quality management, covering data requirements, key metrics, dashboard design principles, and implementation in Power BI, Tableau, and Google Data Studio.

Comprehensive overview of Oracle's role in the pharmaceutical industry, covering their Health Sciences solutions, cloud infrastructure, compliance features, and case studies of successful implementations at major pharma companies.

Practical guide for Market Access teams and field representatives on handling common payer objections in oncology, including strategies for verbal and written communications, real-world examples, and response frameworks.

An in-depth analysis of the leading AI consulting firms serving U.S. pharmaceutical companies, highlighting their focus areas, technologies, and recent activities in drug discovery, compliance, efficiency, and commercial applications.

A comprehensive list of 50+ events and conferences in 2025 that are relevant to Veeva professionals, including Veeva's own conferences, pharma IT events, and industry gatherings, all sorted by relevance score.

A comprehensive analysis of Workday's growing adoption in the pharmaceutical and life sciences sector, examining market trends, key drivers, notable industry users, competitive positioning against SAP, Oracle, and ADP, and future outlook for enterprise cloud solutions in life sciences.

An in-depth exploration of how data science is revolutionizing the life sciences industry, from drug discovery to clinical trials, with real-world applications and case studies. Updated January 2026 with latest FDA AI guidance, Insilico Medicine Phase IIa results, and major industry consolidations.

A comprehensive overview of the most influential open-source software tools transforming pharmaceutical research, development, and manufacturing, from cheminformatics to clinical data management and regulatory compliance.

A detailed technical guide to all APIs available across the Veeva ecosystem, including REST, Bulk APIs, Direct Data API, SDKs, and VAPIL for Vault, Vault CRM, Network, and OpenData. Covers the 2025 Salesforce transition, authentication, versioning, and integration scenarios.

A comprehensive breakdown of Veeva Systems' pricing models, licensing structures, and cost considerations for life sciences companies evaluating Veeva Vault, CRM, and other products.

A comprehensive analysis of Veeva Systems as a long-term investment, examining its market position in life sciences software, FY2026 financial performance, Vault CRM transition, AI initiatives, competitive landscape versus Salesforce, and valuation metrics for 2026 and beyond.

A comprehensive comparison of IQVIA's Orchestrated Customer Engagement (OCE) and Veeva CRM platforms, analyzing features, integrations, usability, support, compliance, security, mobile capabilities, scalability, and user feedback for pharmaceutical organizations.

A comprehensive guide for technical administrators on configuring user-level security in Veeva Vault, covering security profiles, permission sets, roles, and best practices for compliance.

A comprehensive guide to modern data warehousing solutions for life sciences organizations, covering cloud vs. on-premise strategies, technology stacks (Snowflake, Databricks, Redshift, BigQuery, Microsoft Fabric), compliance requirements, and scalable approaches for organizations of all sizes. Updated for 2026 with the latest platform features and market trends.

Practical strategies to boost Veeva CRM adoption among sales reps and MSLs, addressing common challenges like poor user experience, lack of training, and resistance to change.

A comprehensive overview of Customer Relationship Management (CRM) platforms tailored for biotech companies, comparing various solutions and their features for compliance, sales, and customer relationship management in the life sciences sector.

A comprehensive guide to modern healthcare professional (HCP) engagement solutions for pharmaceutical companies, covering omnichannel strategies, compliance requirements, technology platforms, and best practices for tracking and measuring engagement effectiveness.

A comprehensive guide for pharmaceutical marketers on planning and executing market access pull-through campaigns using Veeva CRM and Vault, from stakeholder targeting to field execution. Updated for 2026 with Veeva AI Agents and IRA pricing considerations.

A comprehensive analysis of how pharmaceutical marketing differs from traditional industries, covering regulatory frameworks, key players, allowed practices, and compliance requirements in the U.S. healthcare market.

A comprehensive comparison of CRM requirements between pharmaceutical companies and other life sciences organizations, examining key differences in sales, marketing, compliance, and patient engagement approaches.

A comprehensive comparison of three approaches to adapting large language models for pharmaceutical applications: fine-tuning, distillation, and prompt engineering, with technical details and real-world examples.

A comprehensive analysis of Veeva's decision to end its partnership with Salesforce, exploring the strategic implications, timeline of events, and impact on life sciences companies facing this major industry transition.

A comprehensive overview of the worldwide ecosystem of Veeva consulting partners, highlighting regional specialists and global players in the life sciences sector.
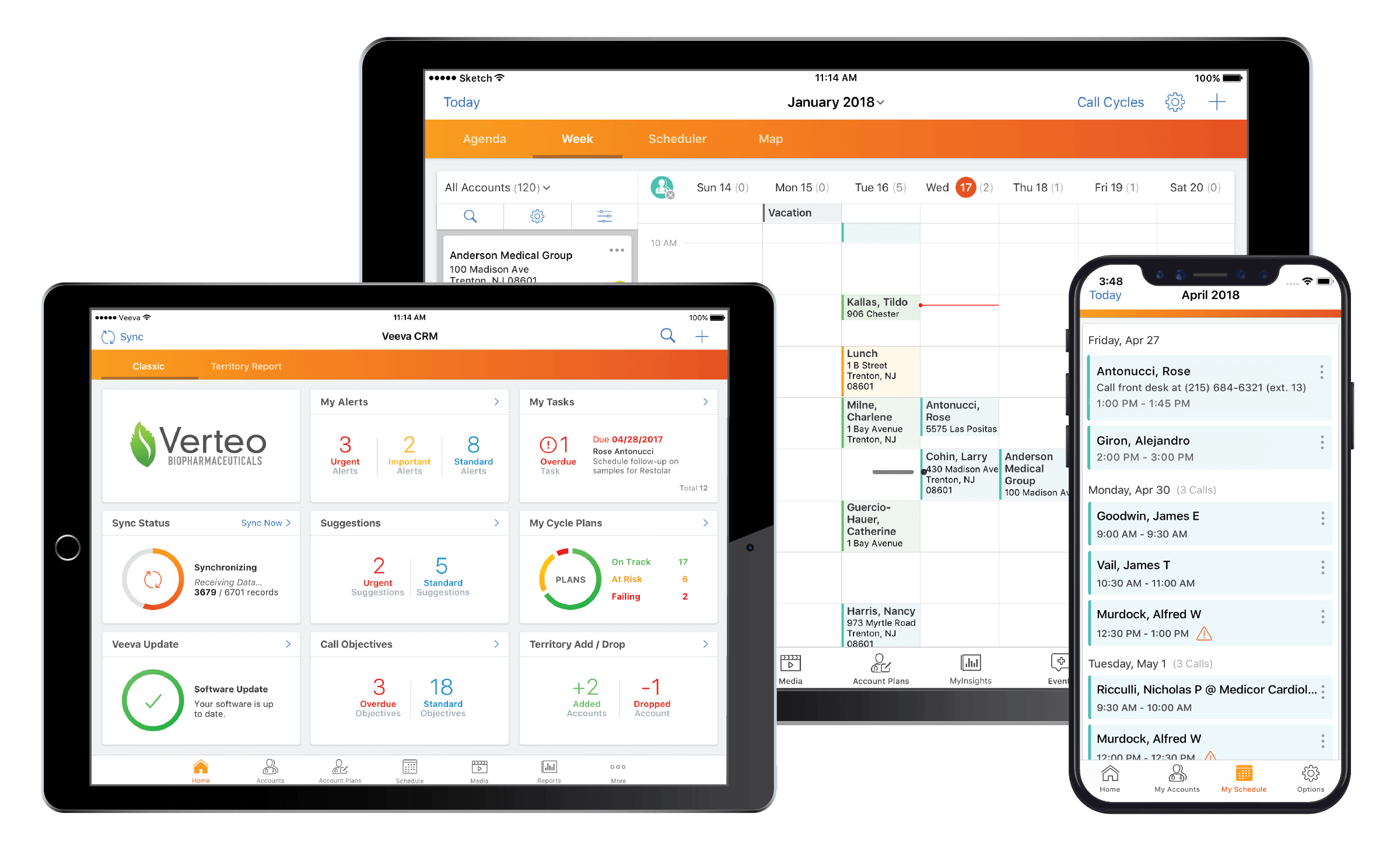
An in-depth analysis comparing Veeva CRM with other CRM solutions in the life sciences industry, examining features, compliance, and industry-specific capabilities.

In-depth analysis of Veeva Systems' transformation from 2021 to 2026, examining strategic shifts, product innovations including AI Agents, Vault CRM adoption, and continued market leadership in life sciences cloud solutions. Updated January 2026.

An in-depth look at Veeva Systems' office locations worldwide, including architecture, workplace culture, team distributions, recent expansions, and future plans for global presence.

A comprehensive overview of Veeva Engage, exploring how this cloud-based platform enables pharmaceutical and life sciences companies to interact with healthcare professionals (HCPs) through compliant digital channels, including virtual meetings, messaging, and content sharing.

A comprehensive technical overview of Veeva Nitro, exploring how this cloud-based data warehouse platform revolutionizes commercial data management and analytics in the pharmaceutical industry through pre-built connectors, industry-specific data models, and integrated analytics capabilities.

A comprehensive overview of Veeva OpenData, exploring how this global customer reference data solution provides pharmaceutical companies with accurate, up-to-date information on healthcare professionals (HCPs) and healthcare organizations (HCOs) to power their commercial operations.

A detailed technical overview of Veeva's Randomization and Trial Supply Management (RTSM) system, covering architecture, features, integration capabilities, and regulatory compliance for clinical trials.

A comprehensive guide to integrating Veeva CRM with SAP Concur, exploring how this integration transforms enterprise workflows by streamlining expense management and ensuring compliance in life sciences.

A comprehensive technical guide to Veeva Vault Platform, covering architecture, development capabilities, integration options, and security features for software developers.
© 2026 IntuitionLabs. All rights reserved.