
Learn best practices for eDiary data collection in clinical trials. This guide covers design, implementation, and regulatory compliance for high-quality PRO dat

Learn best practices for eDiary data collection in clinical trials. This guide covers design, implementation, and regulatory compliance for high-quality PRO dat

A guide to Reference Safety Information (RSI) in clinical trials. Learn the regulatory framework, common pitfalls, and how to avoid SUSAR reporting errors.

Learn what an Investigator's Brochure (IB) is, its required content per ICH GCP guidelines, and its critical role in assessing risk for clinical trials.

A detailed guide to the 13 principles of Good Clinical Practice (GCP). Understand the ICH E6(R2) standards for protecting subjects and ensuring data integrity.

Learn about key clinical trial acronyms. This guide explains the definitions, history, and roles of GCP, ICH, IRB, EDC, and eTMF in clinical research.

Learn the complete data cleaning process in clinical trials. This guide covers clinical data management (CDM) best practices, error detection, and regulatory co
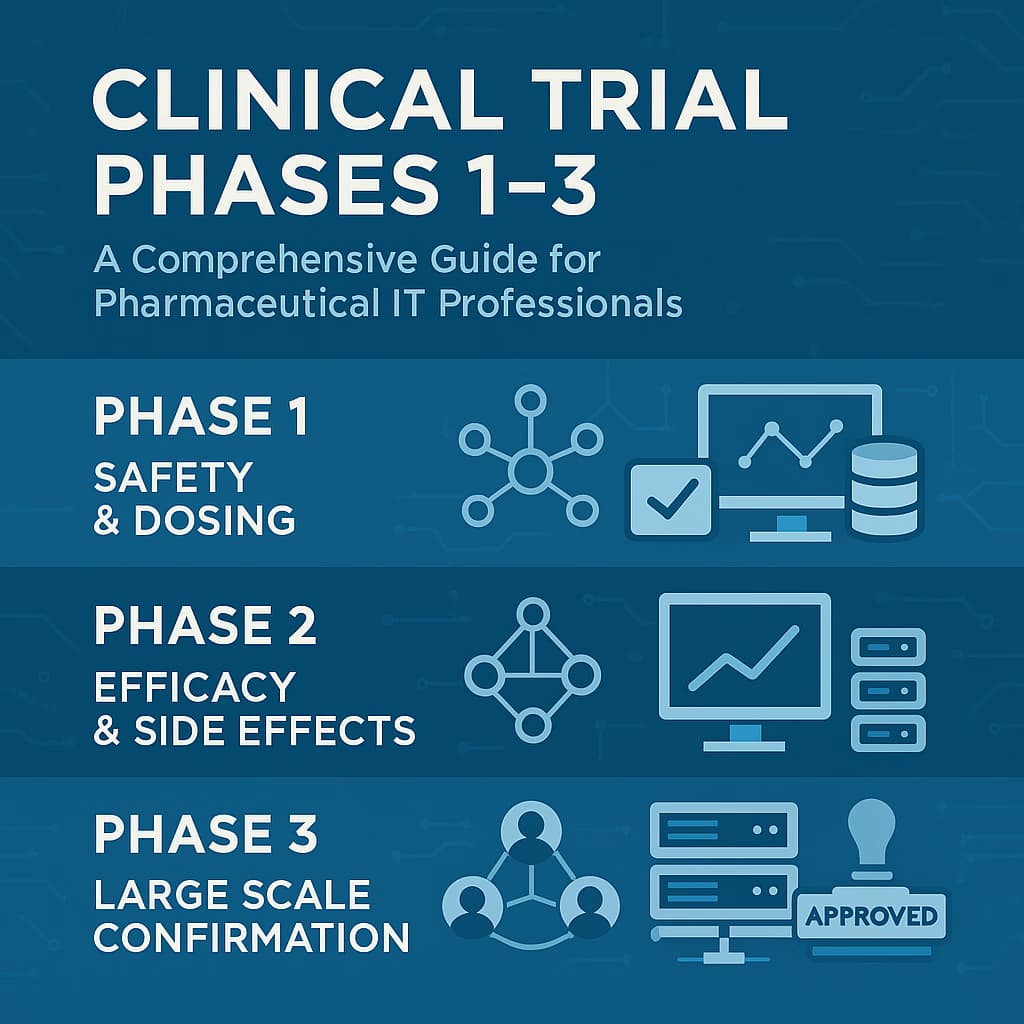
A practical guide to clinical trial phases 1–3 for pharma IT: objectives, compliance, costs, and IT infrastructure for modern drug development.

A comprehensive guide to regulatory requirements and best practices for implementing compliant Randomization and Trial Supply Management (RTSM) systems in clinical trials, covering FDA, EMA, and global standards.

A comprehensive guide to electronic patient record systems in pharmaceutical research and clinical trials, exploring their benefits, implementation challenges, and regulatory considerations.
© 2026 IntuitionLabs. All rights reserved.