
Explore Project Orbis, the FDA's global oncology review framework. Analyze approval timelines, partner agencies, and challenges in expanding beyond cancer.

Explore Project Orbis, the FDA's global oncology review framework. Analyze approval timelines, partner agencies, and challenges in expanding beyond cancer.

Explore AI automation for Clinical Study Reports (CSRs). Analyze efficiency gains, regulatory compliance, and risks like hallucinations and data security.

Explore AI applications in clinical development plans, including protocol optimization, synthetic control arms, and patient recruitment strategies for trials.

A Clinical Development Plan (CDP) outlines the strategy for drug approval. Learn about trial phases, the Target Product Profile, and regulatory requirements.

Analyze when biotechs need pharmacovigilance software. Covers FDA safety database requirements, compliance risks, and solution options for clinical trials.

Analyze AI integration in pharma IT architecture, from R&D to supply chain. Review key data on MLOps, clinical trial efficiency, and FDA guidance.

Analyze the build vs buy AI decision in pharma. Compare costs, risks, and time-to-value for R&D and commercial teams to guide strategic investment.

Efficacy in clinical trials often overstates real-world effectiveness. Learn why this gap exists and how HEOR uses real-world evidence (RWE) to correct for it.

Learn what a Case Report Form (CRF) library is and how it improves clinical trials. This guide covers benefits like data standardization and faster CRF design.

Learn best practices for eDiary data collection in clinical trials. This guide covers design, implementation, and regulatory compliance for high-quality PRO dat

A guide to Reference Safety Information (RSI) in clinical trials. Learn the regulatory framework, common pitfalls, and how to avoid SUSAR reporting errors.

Learn the key differences between ISO 14155 for medical device trials and ICH GCP for pharma. This guide compares their scope, risk management, and regulatory c

An in-depth guide to adaptive trial design. Learn how prespecified changes based on interim data can make clinical trials more efficient and ethical.

An educational guide to sample size calculation in clinical trials. Learn the roles of statistical power, effect size, and alpha/beta errors in trial design.

Explore the Bid Defense Meeting (BDM), a critical step for CROs to win clinical trial contracts. Learn key preparation strategies, sponsor criteria, and best pr

Explore the rise of CRO consolidation in the clinical trials industry. This analysis covers M&A trends, key drivers, and the impact on pharma sponsors and quali

Learn how MedDRA and WHODrug standardize coding for adverse events and medications in clinical trials. This guide covers their structure, use, and best practice

Analyze the future of Contract Research Organizations (CROs) to 2030. Learn about market growth forecasts, the impact of AI, and decentralized clinical trial mo

Explore the evolution from paper CRFs to eCRFs for clinical trials. Compare paper vs. electronic data capture (EDC) on data quality, cost, time, and compliance.

Learn the complete query management process in clinical trials. This guide covers the workflow, high costs, and impact on data integrity for CROs and sites.

Compare ACRP vs. SOCRA clinical research certifications. This guide analyzes eligibility, exam content (ICH GCP), cost, and recertification for CCRC, CCRA & CCR

Learn how to manage protocol deviations in clinical trials. This guide covers classification, reporting, and prevention to ensure data integrity and GCP complia

Learn what an Investigator's Brochure (IB) is, its required content per ICH GCP guidelines, and its critical role in assessing risk for clinical trials.

Learn the essential CDISC standards for clinical trial data. This guide explains the SDTM and ADaM data models, their structure, and use in regulatory submissio

Explore the critical role of an IRB/IEC in protecting human subjects. Learn how ethics committees review and approve clinical trials per key ethical regulations

Learn why patient retention is critical for clinical trial validity. This guide explores the impact of dropouts and provides evidence-based strategies to reduce

A detailed guide to the 13 principles of Good Clinical Practice (GCP). Understand the ICH E6(R2) standards for protecting subjects and ensuring data integrity.

Learn why the drug development timeline averages 10-15 years. This guide details each stage from discovery and preclinical studies to all clinical trial phases

Learn about key clinical trial acronyms. This guide explains the definitions, history, and roles of GCP, ICH, IRB, EDC, and eTMF in clinical research.

Learn the critical differences between preclinical and clinical research in drug development, from lab-based toxicology (GLP) to human clinical trials (GCP).

Explore the distinct roles in clinical trials. Learn the specific responsibilities of sponsors, CROs, and sites, from protocol design to regulatory compliance u

Learn what a Contract Research Organization (CRO) is and its critical role in modern drug development. This guide covers CRO services, benefits, and market tren

An in-depth guide to the four phases of clinical trials. Learn the objectives of Phase I-IV, from establishing drug safety and efficacy to post-approval studies

Learn the complete data cleaning process in clinical trials. This guide covers clinical data management (CDM) best practices, error detection, and regulatory co

A complete guide to clinical trial site close-out. Our checklist covers data integrity, IP accountability, archiving, and regulatory compliance per ICH-GCP & FD

Explore a comprehensive list of the top pharma news websites and biotech publications. Learn about sources for drug development, regulatory changes, and market

Learn how open source software like R and Python is changing pharma R&D. We analyze the shift from proprietary systems to collaborative drug discovery models.

Learn about Veeva Vault eTMF, the leading electronic trial master file solution. Updated for 2026 with AI Agents, TMF RM v4, ICH E6(R3) compliance, and 500+ customers

Explore the key drivers behind major pharma and CRO layoffs in 2025-2026. This analysis covers economic pressures, patent cliffs, and R&D shifts at top companie

Learn what an electronic Investigator Site File (eISF) is and its role in clinical trials. Updated for 2026 with ICH E6(R3) finalization, latest adoption data, and eISF vs. eTMF comparison

Learn about the TMF Reference Model (now TMF Standard Model under CDISC), the industry-standard taxonomy for organizing Trial Master File documents in clinical trials. Covers TMF SM v1, ICH E6(R3) alignment, and AI-powered eTMF automation.

Updated 2026 guide to finding drugs in the clinical pipeline (Phase I-III). Learn to use ClinicalTrials.gov (550K+ studies), CTIS, commercial databases, and AI-powered tools like Pharmaprojects+ and TuneLab.

Explore a detailed cost-benefit analysis of RTSM implementation in clinical trials. Learn how RTSM systems reduce drug waste by 15-30%, save millions, and align with ICH E6(R3) and AI-driven forecasting trends in 2025-2026.

Learn about Patient-Reported Outcomes (PRO) systems in clinical trials. This guide covers ePRO data collection, PROMs, FDA PFDD guidelines, CONSORT 2025 updates, AI-driven ePRO platforms, and challenges

An in-depth analysis of the ICH E6(R3) Good Clinical Practice guidelines for 2025. Explore key changes in Quality by Design, data governance, and decentralized

Updated 2026 guide to synthetic data in pharmaceutical research. Covers acceptance criteria for fidelity, utility, and privacy, plus latest FDA/EMA AI guidance, EHDS, and diffusion model advances.

Learn the end-to-end drug development pipeline, from initial drug discovery and preclinical research to Phase I-IV clinical trials and final FDA approval. Updated for 2026 with latest success rates, AI drug discovery progress, and regulatory developments.

Learn about the specialized software tools used across the drug development lifecycle, from discovery and preclinical research to manufacturing and commercialization. Updated for 2026 with ICH E6(R3), DSCSA compliance deadlines, IDMP/PMS timelines, and the latest in AI-driven drug design.

An explanation of Clinical Data Management (CDM) and its function in research. Learn how CDM ensures high-quality, reliable data for clinical trials.

Examines how AI accelerates the pharmaceutical drug pipeline, reducing time to market. Updated for 2026 with the latest clinical milestones, FDA guidance, and industry developments including Insilico Medicine's Phase IIa results and the Recursion-Exscientia merger.

This article details AI applications in pharmaceutical business intelligence, covering drug discovery, clinical trials, supply chain, real-world evidence, and market intelligence.

Explore key software needs, technology stacks, and specific tools like AI-driven drug design and cheminformatics in pharmaceutical software development.

Explore Medidata Rave CTMS and EDC solutions, including their history, features, and real-world application in clinical trials. Learn about market standing and competitors.

Learn about 10 key AI innovations that optimize clinical trials, improving efficiency, reducing costs, enhancing patient safety, and speeding drug development.

Learn about key technical, regulatory, organizational, ethical, and financial barriers hindering AI adoption in life sciences, with emerging solutions including the latest FDA/EMA guidance and regulatory sandboxes.

Learn why new drug development takes over a decade, discussing the high attrition rates, extensive research, and regulatory hurdles involved in bringing medicines to market.

Learn about leading pharmaceutical market intelligence firms, their data analysis methods, and services like drug pipeline tracking, sales forecasts, and regulatory insights.

This article lists 10 free generative AI courses for pharmaceutical professionals. Learn LLMs, prompt engineering, and AI applications in drug R&D.
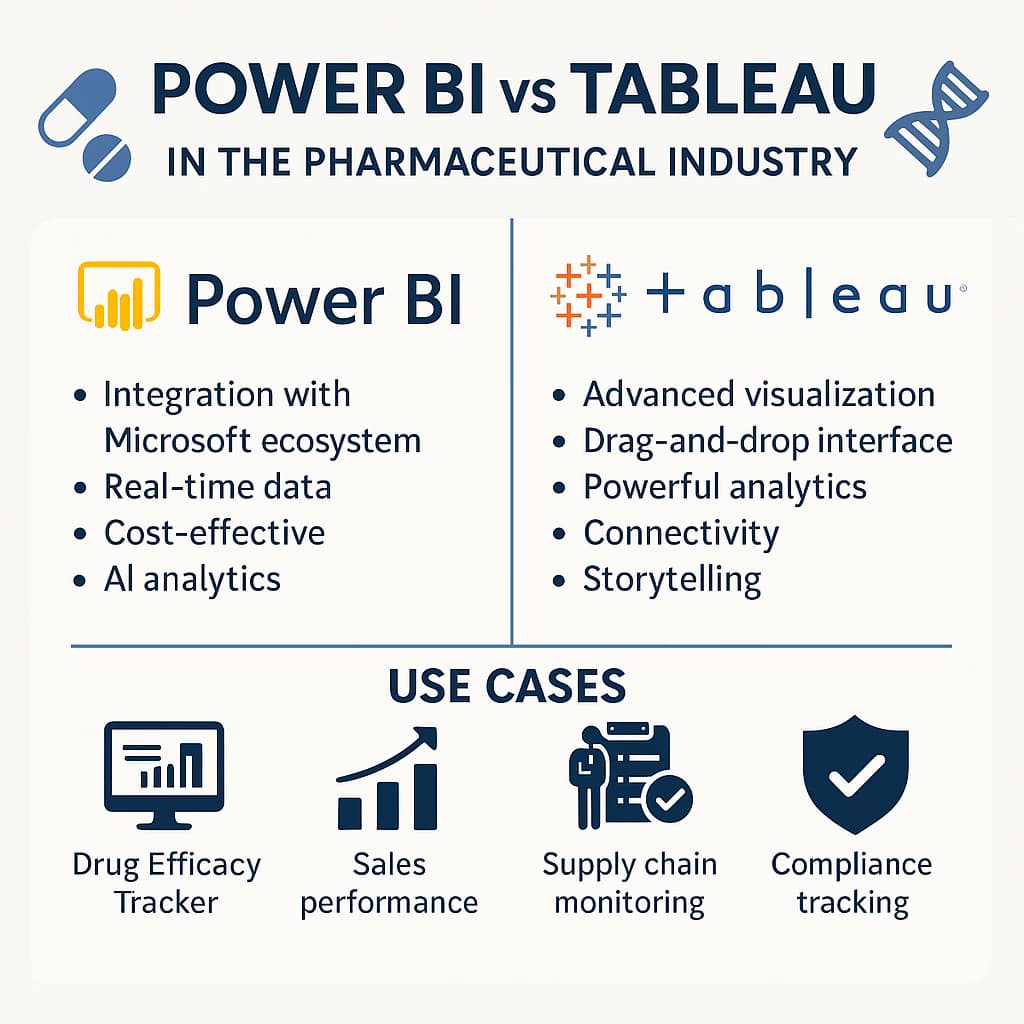
Comprehensive comparison of Power BI and Tableau for pharma: features, pricing, compliance, and use cases for IT and analytics teams.

A technical comparison of AI agents and AI workflows in pharmaceutical IT, with use cases, pros and cons, and adoption trends for U.S. pharma.

An exploration of how artificial intelligence is revolutionizing drug development processes, from target identification to clinical trials, with focus on implementation strategies and success metrics.

Step-by-step guide to all Veeva Vault login methods: username/password, SSO, mobile app, and external/partner access. Updated for 2026 with new MFA, session controls, and 26R1 features. Includes troubleshooting, FAQs, and application-specific notes.

Detailed case studies examining successful implementations of Randomization and Trial Supply Management systems in U.S. clinical trials, highlighting best practices and measurable outcomes.

Exploring how modern RTSM solutions are evolving to improve patient experience in clinical trials, featuring innovative approaches to recruitment, engagement, and trial management.

An in-depth comparison of cloud-based and on-premise Randomization and Trial Supply Management (RTSM) solutions, analyzing security, compliance, cost, and operational considerations for pharmaceutical companies.

An in-depth analysis of how artificial intelligence is transforming clinical data management across US healthcare, from EHR documentation to clinical trials and real-world evidence.

A comprehensive guide to integrating Randomization and Trial Supply Management (RTSM) systems with Electronic Data Capture (EDC) platforms, covering benefits, challenges, and vendor solutions.

An in-depth analysis of top Clinical Research Management Systems in the United States, comparing features, benefits, and implementation strategies for pharmaceutical companies.

Discover how Veeva RTSM's real-time analytics transform clinical trials by optimizing patient randomization, supply management, and operational efficiency with data-driven insights.

Explore how MCP is revolutionizing data integration and AI applications in pharmaceutical research, clinical trials, and healthcare systems for enhanced compliance.

A comprehensive guide to regulatory requirements and best practices for implementing compliant Randomization and Trial Supply Management (RTSM) systems in clinical trials, covering FDA, EMA, and global standards.

A comprehensive guide to how Amazon Web Services (AWS) is transforming pharmaceutical operations from drug discovery to manufacturing, with real-world case studies from Pfizer, Moderna, Merck, and more.

Explore real-world case studies of how pharmaceutical companies are leveraging big data, AI, and cloud computing across the drug lifecycle - from discovery to marketing - with measurable outcomes and lessons learned.

A comprehensive guide to electronic patient record systems in pharmaceutical research and clinical trials, exploring their benefits, implementation challenges, and regulatory considerations.

A comprehensive exploration of generative AI proof of concepts in pharmaceutical research, examining real-world applications, implementation strategies, and measurable outcomes across the drug development pipeline.

A comprehensive analysis of how Google Cloud Platform (GCP) is revolutionizing pharmaceutical operations, from AI-powered drug discovery to clinical trial management and regulatory compliance.

An in-depth exploration of how pharmaceutical companies leverage Microsoft Azure's cloud platform for drug discovery, clinical trials, manufacturing, and regulatory compliance, with real-world case studies and implementation strategies.

Comprehensive guide on RTSM best practices for Phase 3 trials, covering randomization strategies, global supply chain management, regulatory considerations (FDA, ICH E6(R3)), system integration, risk mitigation, and future trends including AI-driven forecasting and decentralized trial support.

Comprehensive analysis of big data technologies used in pharmaceutical industry, including Hadoop, Spark 4.x, cloud data warehouses (Snowflake, Databricks), NoSQL databases, and specialized genomics platforms, with detailed comparisons and implementation examples. Updated for 2025-2026 with latest market data and technology developments.

Comprehensive overview of Oracle's role in the pharmaceutical industry, covering their Health Sciences solutions, cloud infrastructure, compliance features, and case studies of successful implementations at major pharma companies.

A comprehensive analysis of how pharmaceutical companies leverage SAP's enterprise solutions for drug development, clinical trials, manufacturing, supply chain management, and regulatory compliance, with detailed case studies from leading pharma companies.

An in-depth exploration of how data science is revolutionizing the life sciences industry, from drug discovery to clinical trials, with real-world applications and case studies. Updated January 2026 with latest FDA AI guidance, Insilico Medicine Phase IIa results, and major industry consolidations.

A comprehensive overview of the most influential open-source software tools transforming pharmaceutical research, development, and manufacturing, from cheminformatics to clinical data management and regulatory compliance.

An in-depth analysis of the five most digitally innovative pharmaceutical companies in Europe, examining their AI initiatives, digital transformation strategies, and how they're leveraging technology to accelerate drug development and improve patient outcomes.

A detailed analysis of Veeva CTMS features and capabilities, exploring how it streamlines clinical trial operations and improves study execution.

A comprehensive technical analysis of Veeva Vault eTMF, exploring its architecture, key features, and integration capabilities.

A detailed technical overview of Veeva's Randomization and Trial Supply Management (RTSM) system, covering architecture, features, integration capabilities, and regulatory compliance for clinical trials.
© 2026 IntuitionLabs. All rights reserved.