
Learn about the EU AI Act's impact on pharma. Updated for 2026 with the Digital Omnibus proposal, GPAI Code of Practice, and revised Product Liability Directive. Includes risk classification, compliance steps, flowchart & SOP starter kit.

Learn about the EU AI Act's impact on pharma. Updated for 2026 with the Digital Omnibus proposal, GPAI Code of Practice, and revised Product Liability Directive. Includes risk classification, compliance steps, flowchart & SOP starter kit.

Learn to design, secure, and manage a HIPAA-compliant API. Updated for 2026 with the proposed Security Rule overhaul, mandatory encryption and MFA requirements, and current enforcement trends.

An overview of AI applications in the pharmaceutical sector, from generative AI to ML. Explains key IT management challenges like data, compliance, and security.

Explore US pharmaceutical automation compliance, covering FDA regulations like cGMP & 21 CFR Part 11, Pharma 4.0 trends, challenges, and best practices.

Explore how Oracle Cloud CX supports customer experience in life sciences, encompassing CRM, marketing, and service applications for compliant engagement with HCPs and patients.

Learn how regulatory affairs ensures product compliance in health industries. Explore the fundamental role of AI and LLMs in modern regulatory processes, including the latest FDA/EMA joint guidance and EU AI Act requirements.

Evaluate LMS platforms for life sciences. Understand key compliance requirements: FDA 21 CFR Part 11, GxP, electronic records, and audit trails for validated training.

Comprehensive guide to BI and dashboard tools for pharma: Power BI, Tableau, Qlik, Looker, Domo, Sisense, Strategy (MicroStrategy), IBM Cognos. Updated pricing, compliance, and adoption trends for 2025-2026.

How Veeva Crossix and big data are transforming pharma marketing analytics: capabilities, compliance, case studies, and adoption trends in the U.S.

Guide to MarTech API integrations for pharma: CRM, marketing automation, analytics, consent, compliance, and best practices for unified digital engagement.

Guide to outsourcing HCP marketing in pharma: benefits, risks, industry trends, compliance, and how to manage third-party partnerships effectively.

How Veeva Site Connect streamlines sponsor–CRO–site collaboration: features, adoption, compliance, and comparison to Medidata and Oracle Clinical One.

Best practices for pharma teams to secure first meetings with HCPs: outreach tactics, channel comparison, overcoming gatekeepers, and expert tips for success.

A step-by-step career roadmap for pharma IT professionals: from Veeva admin to enterprise architect. Skills, certifications, salary benchmarks, and real-world examples.

Overview of major HCP data providers, U.S. compliance rules, and best practices for pharma IT. Includes vendor comparison, legal requirements, and governance tips.

How biotech and pharma companies use NetSuite for compliance: FDA 21 CFR Part 11, GxP, HIPAA, SOX, audit trails, e-signatures, and reporting best practices.

Evidence-based UX strategies for digital HCP engagement platforms in pharma: trends, challenges, best practices, and compliance for IT teams.
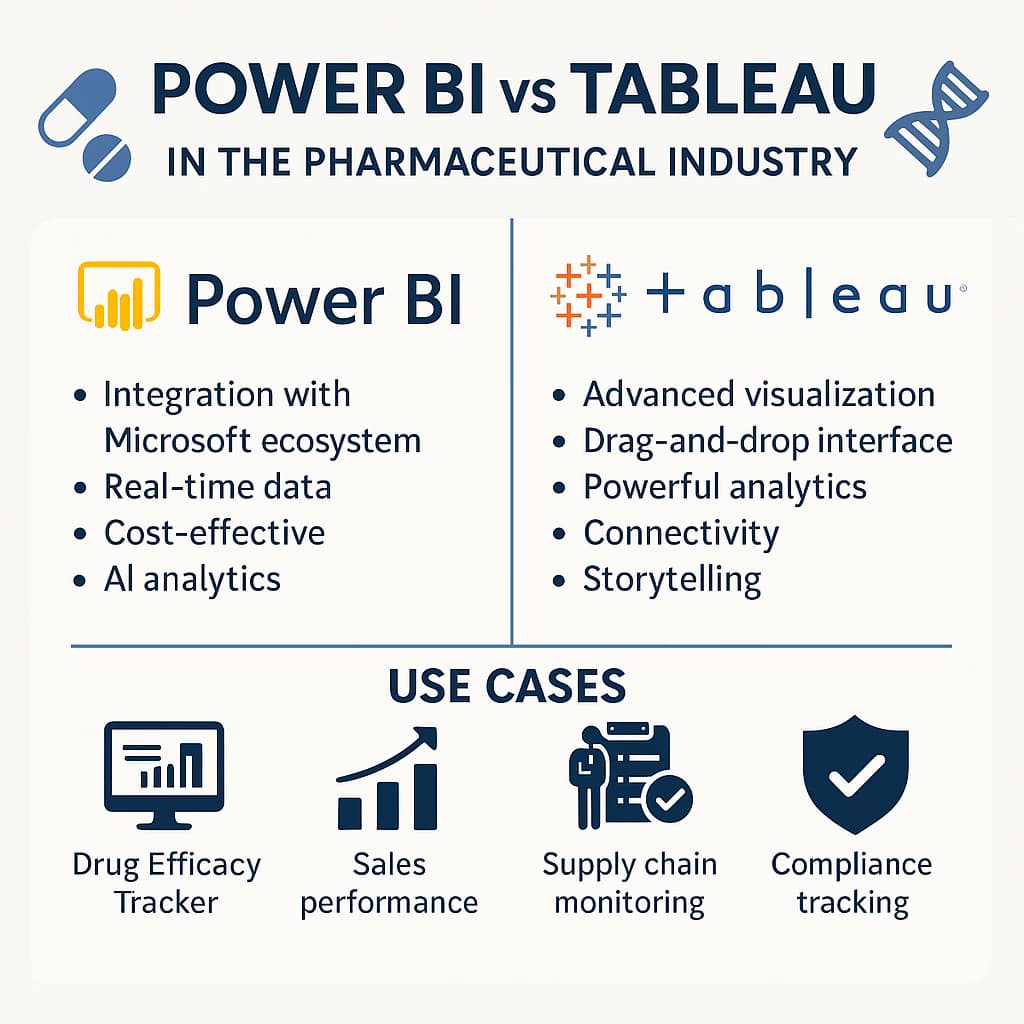
Comprehensive comparison of Power BI and Tableau for pharma: features, pricing, compliance, and use cases for IT and analytics teams.

A technical overview of remote detailing in the pharmaceutical industry: adoption trends, technology platforms, compliance, and best practices.

A comprehensive review of software solutions for optimizing pharmaceutical field sales routes, comparing CRM, SFA, and mapping tools for efficiency, compliance, and analytics.

A detailed comparison of Windsurf (Codeium), Cursor, and GitHub Copilot for enterprise software development in the pharmaceutical industry, focusing on security, compliance, and productivity. Updated January 2026 with latest pricing, features, and the Cognition acquisition of Windsurf.

A comprehensive guide to building and scaling Veeva Vault and CRM data pipelines for terabyte-scale datasets in the pharmaceutical industry, with a focus on compliance and performance.

An analysis of how artificial intelligence is transforming regulatory affairs in pharmaceuticals, from submission preparation to compliance monitoring and regulatory intelligence.

A technical guide to developing customized CRM solutions for pharmaceutical companies using AI-assisted development tools, focusing on compliance, data security, and industry-specific requirements.

A detailed comparison of key compliance frameworks in pharmaceutical IT, including FDA 21 CFR Part 11, GDPR, HIPAA, and GxP, with implementation strategies and best practices.

An in-depth comparison of cloud-based and on-premise Randomization and Trial Supply Management (RTSM) solutions, analyzing security, compliance, cost, and operational considerations for pharmaceutical companies.

Explore how MCP is revolutionizing data integration and AI applications in pharmaceutical research, clinical trials, and healthcare systems for enhanced compliance.

An in-depth guide to designing and implementing modern datacenter infrastructure for pharmaceutical companies, focusing on scalability, security, regulatory compliance, and integration with cloud services for drug development and manufacturing.

A comprehensive analysis of Workday's growing adoption in the pharmaceutical and life sciences sector, examining market trends, key drivers, notable industry users, competitive positioning against SAP, Oracle, and ADP, and future outlook for enterprise cloud solutions in life sciences.

Learn how to identify, segment, and engage Healthcare Professionals (HCPs) and Key Opinion Leaders (KOLs) effectively for pharmaceutical commercialization, including methodologies, data sources, tools, and compliance considerations.

A comprehensive guide to compliant pharmaceutical sales practices, covering FDA regulations, PhRMA Code requirements, and best practices for engaging healthcare professionals while avoiding common compliance pitfalls.

A comprehensive guide to how pharmaceutical companies identify, segment, and engage healthcare professionals (HCPs) for sales outreach, covering databases, targeting strategies, digital tools, and compliance considerations.

A comprehensive guide to Veeva Approved Email and Vault CRM, exploring how pharmaceutical companies use compliant communication tools to engage healthcare providers while adhering to FDA regulations. Updated for 2025-2026 with coverage of Veeva AI Agents, IQVIA OCE+, and the September 2025 FDA enforcement crackdown.

A comprehensive guide for technical administrators on configuring user-level security in Veeva Vault, covering security profiles, permission sets, roles, and best practices for compliance.

A comprehensive guide to modern data warehousing solutions for life sciences organizations, covering cloud vs. on-premise strategies, technology stacks (Snowflake, Databricks, Redshift, BigQuery, Microsoft Fabric), compliance requirements, and scalable approaches for organizations of all sizes. Updated for 2026 with the latest platform features and market trends.

A comprehensive overview of Customer Relationship Management (CRM) platforms tailored for biotech companies, comparing various solutions and their features for compliance, sales, and customer relationship management in the life sciences sector.

A comprehensive guide to modern healthcare professional (HCP) engagement solutions for pharmaceutical companies, covering omnichannel strategies, compliance requirements, technology platforms, and best practices for tracking and measuring engagement effectiveness.

A comprehensive comparison of CRM requirements between pharmaceutical companies and other life sciences organizations, examining key differences in sales, marketing, compliance, and patient engagement approaches.

A comprehensive overview of Veeva Engage, exploring how this cloud-based platform enables pharmaceutical and life sciences companies to interact with healthcare professionals (HCPs) through compliant digital channels, including virtual meetings, messaging, and content sharing.

A comprehensive overview of Veeva OpenData, exploring how this global customer reference data solution provides pharmaceutical companies with accurate, up-to-date information on healthcare professionals (HCPs) and healthcare organizations (HCOs) to power their commercial operations.
© 2026 IntuitionLabs. All rights reserved.