
Learn to implement deep linking in Veeva MyInsights. This technical guide covers key JavaScript API methods, code patterns for ds.newRecord & ds.viewRecord, and

Learn to implement deep linking in Veeva MyInsights. This technical guide covers key JavaScript API methods, code patterns for ds.newRecord & ds.viewRecord, and

A technical guide to troubleshooting Veeva MyInsights on iPad. Learn to diagnose and resolve common sync errors, app crashes, and blank dashboards for pharma CR

Learn to build Veeva Custom Display Elements (CDEs) for MyInsights & X-Pages using React or Vue. This guide covers CDE architecture and development workflow.

Learn the complete MyInsights Studio formula syntax for Veeva CRM. This guide covers calculated fields, data elements, query filters, and expressions with examp

An in-depth guide to the Veeva MyInsights JavaScript API. Learn to use the ds object and core methods like runQuery to query CRM data for custom dashboards.

Learn about value-based contracting (VBC) in pharma. Our guide covers how these agreements tie drug reimbursement to patient outcomes, key data requirements, an

An educational guide comparing the EU Risk Management Plan (RMP) vs. the US REMS for drug safety. Learn about key differences in scope, content, and triggers.

Learn to implement an audit-ready ISO 27001 ISMS for life sciences. This guide covers integrating cybersecurity with GxP, QMS, and FDA regulatory compliance.
Learn the key differences between LIMS, ELN, SDMS, and CDS. This guide explains the purpose, features, and use cases for each lab informatics system.

A clear comparison of the DSUR vs. the PSUR/PBRER. Understand the distinct purpose, scope, timing, and governance of these key pharmacovigilance safety reports.
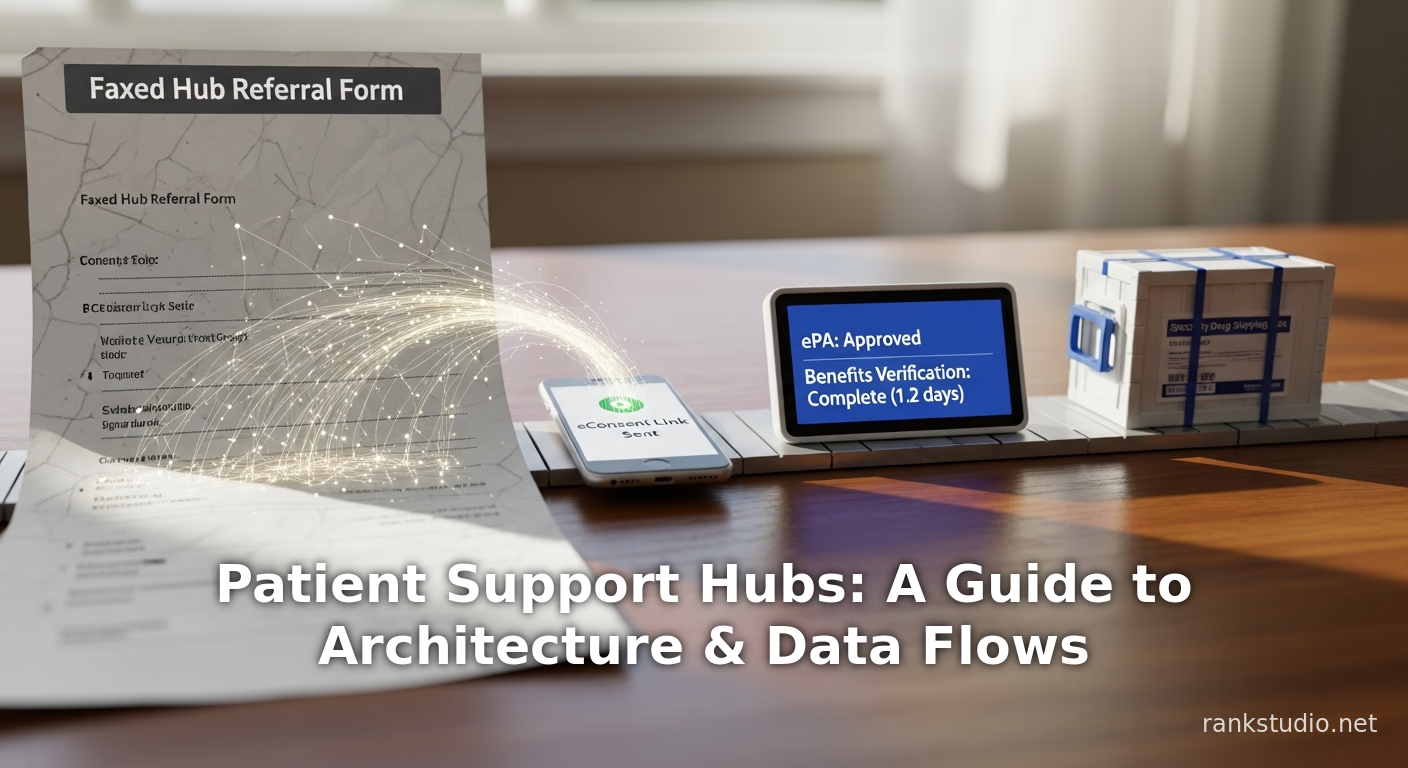
Learn about patient support hub programs in specialty pharma. This guide covers key workflows, technology, data management, and compliance for improving drug ac

An in-depth guide to HTA dossiers. Explore the evidence requirements, economic models, and submission templates for NICE (UK), CADTH (Canada), and ICER (US).

An in-depth guide to the ICH E2B(R3) standard for pharmacovigilance. Explore the data structure of ICSRs, electronic reporting rules, and key global systems.

An in-depth analysis of the top pharmacovigilance databases: Oracle Argus, ArisGlobal LifeSphere, and Veeva Vault Safety. Compare features, compliance & costs.
Explore Gross-to-Net (GTN) revenue management in pharma. Compare leading GTN software vendors Model N, Vistex, and Revitas on features, compliance, and integrat

Compare leading patient hub services platforms. This detailed analysis of CareMetx, AssistRx, Phil, and Lash Group covers their technology, services, and metric

Analyze leading pharmaceutical artwork management systems. This guide compares Esko, Kallik, and Blue Software for pharma label compliance, workflows, and audit

Choosing a GxP ELN? Compare Benchling, IDBS, and LabArchives on features for 21 CFR Part 11 compliance, system validation, and ALCOA data integrity principles.

Learn the compliant process for answering off-label HCP inquiries. This guide details FDA guidance, medical information SOPs, and software for case management.

Learn how pharma firms use competitive intelligence software to mitigate patent cliff risks. We analyze strategies for managing drug patent expiration and reven

Efficacy in clinical trials often overstates real-world effectiveness. Learn why this gap exists and how HEOR uses real-world evidence (RWE) to correct for it.

Learn about outcomes-based drug reimbursement contracts. This article explains the complex IT systems & data infrastructure needed to track real-world patient o

An educational guide to QALYs and ICERs, the core metrics in health economics. Learn how they measure value and guide cost-effectiveness decisions.

An in-depth comparison of ICER (US) vs NICE (UK). Explore their methods, QALY thresholds, and how they assess drug value to influence global pricing floors.
© 2026 IntuitionLabs. All rights reserved.