
Best practices for pharma teams to secure first meetings with HCPs: outreach tactics, channel comparison, overcoming gatekeepers, and expert tips for success.

Best practices for pharma teams to secure first meetings with HCPs: outreach tactics, channel comparison, overcoming gatekeepers, and expert tips for success.

A step-by-step career roadmap for pharma IT professionals: from Veeva admin to enterprise architect. Skills, certifications, salary benchmarks, and real-world examples.

Overview of major HCP data providers, U.S. compliance rules, and best practices for pharma IT. Includes vendor comparison, legal requirements, and governance tips.

How biotech and pharma companies use NetSuite for compliance: FDA 21 CFR Part 11, GxP, HIPAA, SOX, audit trails, e-signatures, and reporting best practices.

Evidence-based UX strategies for digital HCP engagement platforms in pharma: trends, challenges, best practices, and compliance for IT teams.
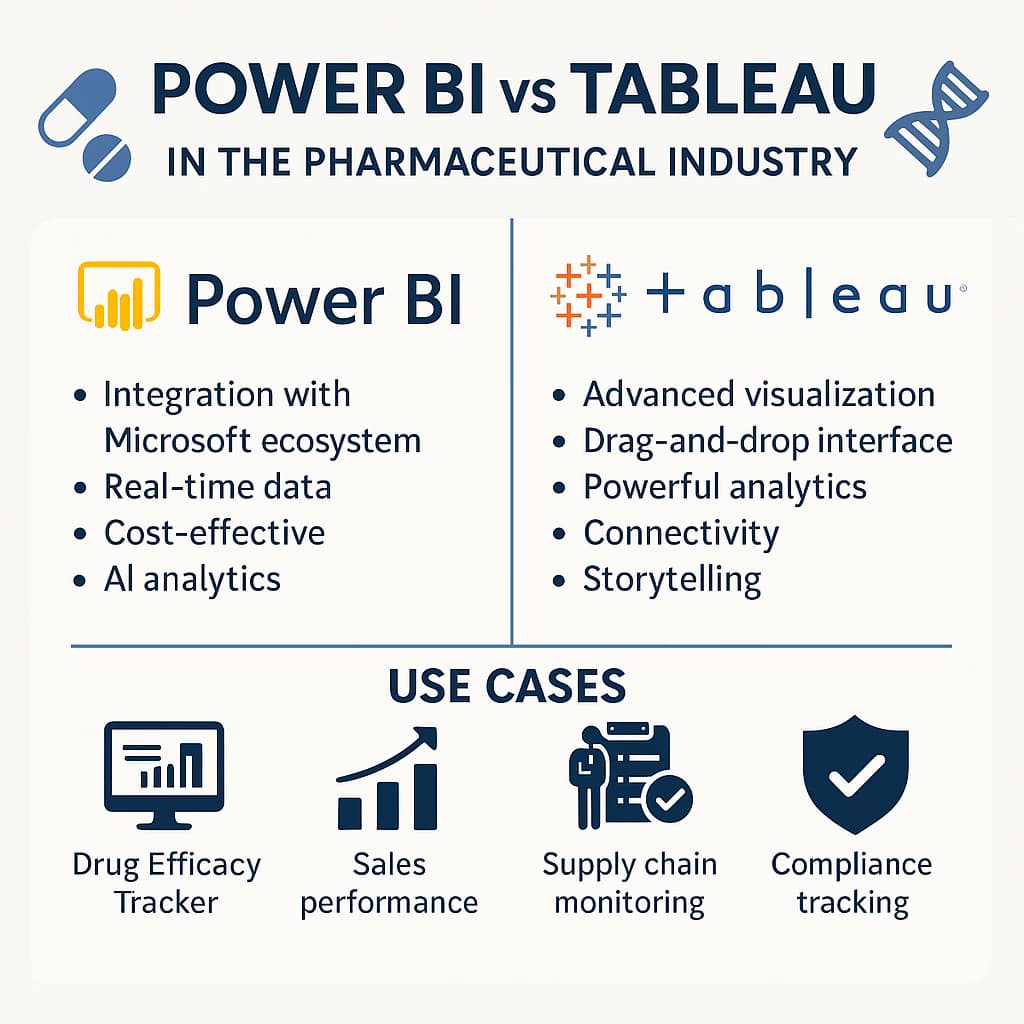
Comprehensive comparison of Power BI and Tableau for pharma: features, pricing, compliance, and use cases for IT and analytics teams.

A technical comparison of AI agents and AI workflows in pharmaceutical IT, with use cases, pros and cons, and adoption trends for U.S. pharma.

A technical overview of remote detailing in the pharmaceutical industry: adoption trends, technology platforms, compliance, and best practices.

A detailed survey of large language model benchmarks in life sciences, covering biomedical NLP, drug discovery, and genomics, with industry use cases and top model performance.

A comprehensive review of software solutions for optimizing pharmaceutical field sales routes, comparing CRM, SFA, and mapping tools for efficiency, compliance, and analytics.

A detailed comparison of Windsurf (Codeium), Cursor, and GitHub Copilot for enterprise software development in the pharmaceutical industry, focusing on security, compliance, and productivity. Updated January 2026 with latest pricing, features, and the Cognition acquisition of Windsurf.

A comprehensive guide to building and scaling Veeva Vault and CRM data pipelines for terabyte-scale datasets in the pharmaceutical industry, with a focus on compliance and performance.

An exploration of how artificial intelligence is revolutionizing drug development processes, from target identification to clinical trials, with focus on implementation strategies and success metrics.

An analysis of how artificial intelligence is transforming regulatory affairs in pharmaceuticals, from submission preparation to compliance monitoring and regulatory intelligence.

Step-by-step guide to all Veeva Vault login methods: username/password, SSO, mobile app, and external/partner access. Updated for 2026 with new MFA, session controls, and 26R1 features. Includes troubleshooting, FAQs, and application-specific notes.

A technical guide to developing customized CRM solutions for pharmaceutical companies using AI-assisted development tools, focusing on compliance, data security, and industry-specific requirements.

Detailed case studies examining successful implementations of Randomization and Trial Supply Management systems in U.S. clinical trials, highlighting best practices and measurable outcomes.

A comprehensive analysis of ChatGPT integration in life sciences, examining implementation strategies, regulatory compliance, and real-world applications across pharmaceutical research and development.

An in-depth analysis of the biotech ecosystem in the San Francisco Bay Area, examining key players, emerging startups, investment trends, and innovation clusters as of January 2026. Updated with latest data on 23andMe bankruptcy, major acquisitions, and 2025 layoffs.

Exploring how modern RTSM solutions are evolving to improve patient experience in clinical trials, featuring innovative approaches to recruitment, engagement, and trial management.

A detailed comparison of key compliance frameworks in pharmaceutical IT, including FDA 21 CFR Part 11, GDPR, HIPAA, and GxP, with implementation strategies and best practices.
An evaluation of RAG systems' effectiveness in processing pharmaceutical documentation, analyzing accuracy, compliance adherence, and practical applications in drug development and clinical trials.

An in-depth comparison of cloud-based and on-premise Randomization and Trial Supply Management (RTSM) solutions, analyzing security, compliance, cost, and operational considerations for pharmaceutical companies.

A comprehensive analysis of the remote patient monitoring landscape in the US healthcare system, examining technological advances, regulatory framework, and implementation challenges in 2025.
© 2026 IntuitionLabs. All rights reserved.