
An educational summary of late 2025 AI research papers. Learn about advances in foundation models like GPT-5, agentic AI, and new neuromorphic hardware.

An educational summary of late 2025 AI research papers. Learn about advances in foundation models like GPT-5, agentic AI, and new neuromorphic hardware.

Learn what reinforcement learning (RL) is through clear explanations and examples. This guide covers core concepts like MDPs, agents, rewards, and key algorithm

Learn what SharePoint Framework (SPFx) is, the client-side model for M365 development. Explore its history, architecture, and use with AI coding agents like Cla

A technical analysis of Google TPU architecture, from v1 to v7. Learn how this custom AI accelerator powers Gemini 3 with superior performance and efficiency vs

Learn why Medical Science Liaisons (MSLs) can discuss off-label data via scientific exchange while sales reps cannot due to strict FDA promotion regulations.

Learn how to create a Unified HCP View in Veeva Vault. This article provides an architectural blueprint for integrating commercial, medical, and clinical data f

A comprehensive analysis of how data quality and data culture are foundational to AI success in pharmaceutical and life sciences organizations, covering assessment frameworks, governance models, regulatory compliance, and practical implementation roadmaps.

An analysis of private equity value creation for TPA & PBM companies post-acquisition. Learn key strategies for growth, operational efficiency, and M&A integrat
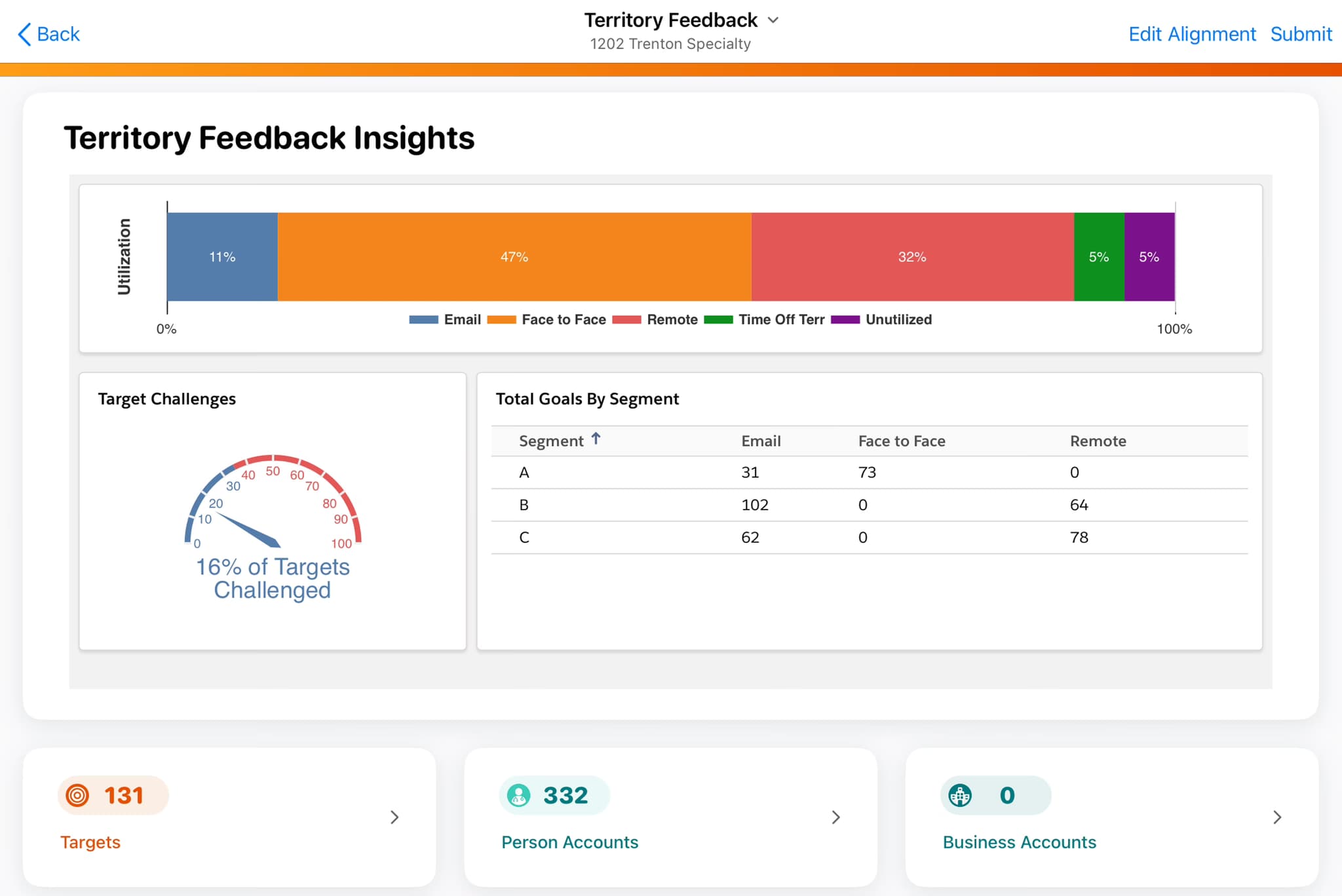
Complete guide to Veeva X-Pages development services for Vault CRM. Covers X-Pages Studio vs custom development, timelines, costs, and how to select a development partner.
Learn the 5-stage eQMS maturity model for Biotech & MedTech startups. This framework guides QMS implementation for FDA and ISO 13485 compliance and scalability.

This 2026 analysis reviews top patient payment platforms for automating collections. Compare features and performance of PatientPay, Cedar, RevSpring, and more.

Explore a detailed comparison of cash flow forecasting software for health systems. This 2026 analysis reviews StrataJazz, Obol, and Centage for hospital financ

A data-driven analysis of 2025 KLAS scores for top enterprise PACS vendors. Compare Sectra, Agfa, Fujifilm, and others on user satisfaction and performance.

Compare top healthcare CMMS platforms. This analysis reviews Accruent TMS, Brightly Biomed, and TRIMEDX RSQ on features, compliance, ROI, and user experience.

Learn the SCDC framework for differentiated messaging of high-value AI solutions in life sciences. This guide explains how to tailor content for diverse stakeho

Explore a 2026 ROI analysis of Quality 4.0 in pharma manufacturing. Learn how technologies like AI & digital twins drive up to 45% cost savings and 40% capacity

Learn what a Case Report Form (CRF) library is and how it improves clinical trials. This guide covers benefits like data standardization and faster CRF design.

Explore OpenStudyBuilder, the open-source platform for clinical study specifications. This deep dive explains its metadata-driven architecture and use of CDISC

Analyze ServiceNow use cases in healthcare and life sciences. This guide covers ITSM for clinical workflows, patient experience, and compliance (HIPAA, GxP) wit

An in-depth analysis of OpenAI's GPT-5.2 achieving a 54% score on the ARC-AGI-2 benchmark for abstract reasoning. Learn how it compares to Gemini 3 & humans.
Explore the modern biotech lab's evolution. Learn how automation, AI, cloud labs, and digital tools are transforming research, from high-throughput screening to

An analysis of the Agentic AI Foundation (AAIF) by the Linux Foundation. Understand its mission to create open standards for agentic AI and prevent vendor lock-

Explore document automation for pharma. Learn how template-driven assembly and structured content, inspired by the tech sector, can cut authoring time for study

Learn how Retrieval-Augmented Generation (RAG) connects ELN, LIMS, and institutional knowledge for drug discovery. This guide explains RAG systems for pharma R&