Videos
Browse videos by topic
All Videos
Showing 961-984 of 1435 videos

Kimberly McElman | Ask an Expert: eConsent Demo with Q&A - Register for Veeva Summit on October 14
Veeva Systems Inc
/@VeevaSystems
Oct 4, 2021
This video serves as a promotional announcement for a critical session held at the Veeva R&D and Quality Summit, focusing specifically on the digital transformation of clinical trial processes through electronic informed consent (eConsent). Hosted by Kimberly McElman, the session, titled "Ask an Expert: eConsent Demo with Q&A," is designed to provide a comprehensive, practical demonstration of Veeva’s specialized tools for managing patient consent in a regulated environment. The core components highlighted for discussion are the functionalities of the Veeva eConsent Editor and the detailed patient workflow facilitated by the MyVeeva for Patients platform. The session’s focus on the Veeva eConsent Editor is highly significant, as this tool is central to ensuring that clinical trial sponsors and Contract Research Organizations (CROs) can efficiently author and deploy compliant consent forms. The demonstration is expected to cover features vital for regulatory adherence, such as dynamic content generation based on study arm or patient demographics, robust version control necessary for audit trails, and the ability to manage multi-language consent forms across global trials. For technology providers, understanding the editor's architecture is key to developing integrated AI solutions, such as automated regulatory text analysis or compliance validation agents that operate within the Veeva ecosystem. Furthermore, the emphasis on the patient e-consent process within MyVeeva for Patients underscores the industry's shift toward decentralized and patient-centric trials. The session will likely illustrate how patients interact with the digital consent forms—accessing documents remotely, utilizing interactive elements (like educational videos or comprehension checks), and providing legally binding electronic signatures. This workflow is critical for optimizing patient engagement and reducing dropout rates. By analyzing this process, firms can identify strategic points to deploy AI-powered enhancements, such as LLMs to simplify complex medical jargon or intelligent chatbots to answer common patient questions about the consent document, thereby improving clarity and ensuring true informed consent while maintaining GxP compliance. Key Takeaways: • **Veeva Platform Centrality:** The video confirms the continued dominance of Veeva Systems in the clinical operations space, specifically in R&D and Quality domains, validating the strategic importance of specializing in Veeva consulting and integration services for pharmaceutical clients. • **Regulatory Compliance Focus:** The context of eConsent mandates strict adherence to regulations like 22 CFR Part 11 regarding electronic signatures and records. The session will detail how Veeva’s solution ensures data integrity, auditability, and non-repudiation, which is a core requirement for any custom software or AI solution integrated into this workflow. • **MyVeeva for Patients Integration:** Understanding the technical and user experience aspects of MyVeeva for Patients is crucial, as this platform dictates the patient interface for clinical data and consent. This knowledge allows for the development of patient-facing AI tools that seamlessly integrate into the existing Veeva environment without disrupting validated processes. • **Optimization of Clinical Trial Start-up:** Effective eConsent solutions drastically reduce the time and administrative burden associated with paper-based processes. Insights from the demo can inform consulting strategies aimed at optimizing clinical operations by leveraging digital tools for faster site activation and patient enrollment. • **AI Opportunities in Content Management:** The Veeva eConsent Editor manages large volumes of complex, regulated text. This presents a prime opportunity for Generative AI and LLMs to assist in drafting localized consent forms, summarizing complex protocol details for site staff, or automatically flagging potential compliance risks in draft documents. • **Data Pipeline Requirements:** The implementation of eConsent generates structured, high-value data (e.g., consent status, version history, time stamps). This necessitates robust data engineering services to integrate this information into broader clinical data warehouses and business intelligence dashboards for real-time operational insights. • **Interoperability with Veeva Vault:** Since eConsent is part of the larger Veeva Vault Clinical Suite, the session implicitly highlights the need for solutions that ensure seamless interoperability with other modules, such as Veeva eTMF (electronic Trial Master File) and CTMS (Clinical Trial Management System), to maintain a unified source of truth. • **Validation of Digital Strategy:** The high-profile nature of the session at the R&D and Quality Summit confirms that digital consent is no longer a niche technology but a standard requirement for modern, compliant clinical trials, reinforcing the strategic importance of offering specialized digital solutions in this area. Tools/Resources Mentioned: * Veeva eConsent Editor * MyVeeva for Patients * Veeva R&D and Quality Summit Key Concepts: * **eConsent (Electronic Informed Consent):** The process of obtaining and documenting a patient's voluntary agreement to participate in a clinical trial using digital methods, ensuring compliance with regulatory standards for electronic records and signatures. * **MyVeeva for Patients:** A component of the Veeva platform designed to enhance patient engagement and communication throughout a clinical trial, serving as the interface through which patients often access and sign eConsent forms.

Alice Foltyn | Vault CDMS to Clinical Operations Connection - Register for Summit on October 14
Veeva Systems Inc
/@VeevaSystems
Oct 4, 2021
This video provides an introductory overview of a specialized session presented by Alice Foltyn at the Veeva R&D and Quality Summit, focusing on the critical integration point between Veeva Vault Clinical Data Management Suite (CDMS) and Veeva Vault Clinical Operations (ClinOps). The primary objective of the session is to demonstrate how connecting these two essential components of the clinical trial ecosystem can significantly boost productivity. The discussion centers on leveraging the "Vault Unified" experience—a foundational concept within the Veeva platform—to manage complex, bi-directional data flows seamlessly, thereby bridging the gap between study management activities and the actual collection and cleaning of clinical data. The core mechanism explored is the establishment of robust, bi-directional data pipelines between the CDMS and ClinOps vaults. In traditional clinical environments, data often resides in silos, requiring manual reconciliation or complex, custom integrations between the EDC (Electronic Data Capture, often handled by CDMS) and the CTMS (Clinical Trial Management System, handled by ClinOps). By utilizing the native integration capabilities of the Vault Unified platform, the system can automatically synchronize key information. This synchronization allows operational teams (ClinOps) to have real-time visibility into data collection progress, query status, and data quality metrics managed by the data management teams (CDMS). Conversely, data management teams benefit from immediate access to operational metrics, such as site activation status, patient enrollment figures, and monitoring visit schedules, which are crucial for planning data cleaning cycles and resource allocation. The ultimate goal of this integration is the streamlining of end-to-end clinical processes. By connecting study management—which encompasses planning, site monitoring, and regulatory document management—with clinical data activities—such as CRF design, data entry, query management, and database lock—organizations can achieve faster cycle times and reduce the risk of errors associated with manual data transfer and reconciliation. This unified approach directly supports faster data review cycles, more efficient source data verification (SDV) planning, and improved oversight of the entire trial lifecycle, reinforcing the value proposition of a fully integrated, compliant technology stack for life sciences companies. Key Takeaways: • **Achieving True Clinical Unification:** The session emphasizes that connecting Vault CDMS and Vault ClinOps is essential for realizing the full benefits of the Veeva Vault platform, moving beyond siloed systems to a single source of truth for both operational and clinical data. • **Enabling Bi-directional Data Flow:** The integration facilitates the automatic exchange of data points, ensuring that updates made in the CDMS (e.g., data query status) are immediately reflected in ClinOps (e.g., monitoring reports), and vice versa (e.g., site visit schedules impacting data review timelines). • **Boosting Clinical Productivity:** By automating the data exchange between these two critical functions, organizations can drastically reduce manual effort required for data reconciliation, status tracking, and reporting, leading to faster trial execution and database lock. • **Real-time Operational Visibility:** ClinOps teams gain immediate, accurate insight into the progress of data collection and cleaning, allowing for proactive adjustments to monitoring schedules, resource deployment, and risk-based quality management (RBQM) strategies. • **Streamlining Study Management:** The connection simplifies the management of clinical trials by linking the study protocol and site details (managed in ClinOps) directly to the data capture forms and cleaning processes (managed in CDMS). • **Enhanced Regulatory Compliance:** A unified system simplifies audit trails and ensures data integrity, as all operational and clinical data are managed within a single, compliant, validated environment, crucial for meeting GxP and 21 CFR Part 11 requirements. • **Optimizing Source Data Verification (SDV):** Real-time data quality metrics from CDMS can inform ClinOps teams precisely where and when SDV efforts should be focused, enabling a more efficient and targeted approach to monitoring. • **Foundation for AI/LLM Solutions:** The establishment of clean, unified data pipelines between CDMS and ClinOps is a prerequisite for deploying advanced AI and LLM tools, such as intelligent automation for query generation or predictive analytics for trial risk assessment. • **Strategic Consulting Opportunity:** For consulting firms, this integration point represents a high-value service area, requiring specialized expertise in both Veeva Clinical Suite configuration and data engineering to ensure optimal bi-directional flow setup and performance tuning. Tools/Resources Mentioned: * **Veeva Vault CDMS (Clinical Data Management Suite):** The platform used for electronic data capture (EDC), data cleaning, query management, and database lock. * **Veeva Vault Clinical Operations (ClinOps):** The suite used for managing clinical trial processes, including CTMS (Clinical Trial Management System), eTMF (electronic Trial Master File), and study start-up. * **Vault Unified:** The overarching framework that allows different Veeva Vault applications to communicate and share data seamlessly, eliminating data silos. Key Concepts: * **Bi-directional Data Flows:** The continuous, automated exchange of information between two systems, where data changes in System A are reflected in System B, and changes in System B are reflected back in System A. * **CDMS to ClinOps Connection:** The specific integration focusing on linking clinical data collection and cleaning activities with operational management activities like monitoring, site visits, and regulatory document tracking.

Sharmin Nasrullah | Demo: Advanced Study Builds in Vault EDC - Register for Summit on October 14
Veeva Systems Inc
/@VeevaSystems
Oct 4, 2021
This announcement previews a demonstration session focused on leveraging Veeva Vault EDC (Electronic Data Capture) for building complex and modern clinical study designs. Presented by Sharmin Nasrullah, Solution Consulting Manager at Veeva, the session is positioned within the context of the Veeva R&D and Quality Summit, highlighting the platform’s capabilities in the critical area of clinical data management and operations. The core value proposition of the demonstration is showing how Vault EDC can implement intricate study protocols—which often include adaptive design elements, decentralized components, or master protocols—with enhanced speed and user ease. The emphasis on "Advanced Study Builds" suggests that Veeva is addressing the growing complexity of clinical trials in the pharmaceutical and biotech sectors. Traditional EDC systems often struggle to accommodate dynamic changes, complex randomization schemes, or the integration of diverse data sources (e.g., wearables, labs, ePRO). Vault EDC’s approach likely focuses on configuration flexibility, reusable design components, and streamlined workflow management, allowing clinical operations teams and Contract Research Organizations (CROs) to rapidly translate sophisticated protocol requirements into a functional, validated study environment. This capability is essential for accelerating trial timelines and reducing the administrative burden associated with mid-study amendments. For specialized consulting firms, the session underscores the necessity of deep expertise in the Veeva Vault Clinical Suite. The ability to implement complex designs "with speed and ease" implies advanced knowledge of Vault EDC’s configuration tools, including form design, edit checks, data mapping, and integration points with other Vault applications like eTMF and CTMS. Furthermore, the focus on R&D and Quality mandates that these advanced study builds adhere strictly to GxP standards and regulatory requirements, ensuring that the speed of implementation does not compromise data integrity or audit readiness. This platform evolution creates significant opportunities for AI and data engineering services to build robust, compliant data pipelines that can efficiently handle the non-uniform and high-velocity data generated by these modern, complex trial structures. Key Takeaways: * **Focus on Modern Trial Design:** The demonstration confirms Veeva’s commitment to supporting complex clinical trial methodologies, such as adaptive trials, master protocols (umbrella/basket), and decentralized clinical trials (DCTs), which require dynamic data capture forms and flexible workflow logic within the EDC system. * **Efficiency in Configuration:** The promise of implementing complex designs with "speed and ease" suggests significant improvements in Vault EDC's configuration interface, likely involving low-code/no-code tools, template libraries, and enhanced version control for study amendments. * **Impact on Clinical Operations:** Rapid study build capabilities directly reduce the time from protocol finalization to site activation, a critical metric for pharmaceutical R&D departments seeking to accelerate drug development timelines. * **Data Structure Complexity:** Advanced study designs inherently lead to more complex and varied data structures. Data engineering teams must develop flexible, scalable pipelines capable of handling non-uniform clinical data and integrating diverse data sources originating from the Vault EDC environment. * **Regulatory Compliance in R&D:** The context of the Veeva R&D and Quality Summit highlights the necessity of ensuring that rapid study builds maintain strict regulatory compliance (e.g., GxP, 21 CFR Part 11). Consultants must ensure that all configurations are validated and that audit trails are robustly maintained. * **Integration with Veeva Ecosystem:** Successful implementation of advanced study builds requires seamless integration between Vault EDC and other components of the Veeva Clinical Operations suite (e.g., Vault CTMS for site management and Vault eTMF for document control), ensuring data consistency across the clinical lifecycle. * **AI Opportunity in Data Quality:** The complexity of the data mandates advanced quality checks. AI/ML solutions can be deployed post-ingestion to monitor data integrity, identify anomalies, and automate source data verification (SDV) processes more efficiently than traditional manual methods. * **Strategic Consulting Requirement:** Companies need specialized consulting expertise not just in Veeva configuration, but in translating complex scientific protocols into optimized, compliant EDC system builds, maximizing the return on investment in the Vault platform. Tools/Resources Mentioned: * Veeva Vault EDC (Electronic Data Capture) * Veeva R&D and Quality Summit Key Concepts: * **Vault EDC:** A cloud-based application within the Veeva Vault platform used for capturing, managing, and cleaning clinical trial data. It serves as the primary data repository for clinical studies. * **Advanced Study Builds:** Refers to the configuration and setup of clinical trials that employ complex methodologies, such as adaptive designs (where the protocol changes based on interim data), master protocols (like umbrella or basket trials), or trials incorporating decentralized elements (DCTs). * **R&D and Quality Summit:** An industry event focused on technology and best practices within pharmaceutical research, development, and regulatory compliance, indicating a focus on GxP and validated processes.

Sharmin Nasrullah | Vanderbilt Shares Insight on the Site Experience - Register for Summit on Oct 14
Veeva Systems Inc
@VeevaSystems
Oct 4, 2021
This video serves as a promotional announcement for a critical panel session scheduled for the upcoming Veeva R&D and Quality Summit. Presented by Sharmin Nasrullah, a Solution Consulting Manager at Veeva, the session focuses on gathering direct insights from clinical sites regarding their operational experience with Electronic Data Capture (EDC) systems, specifically Veeva Vault EDC. The primary goal of the panel is to illuminate how technology impacts the daily workflow of clinical research professionals and how platforms like Vault EDC can simplify complex processes. The central theme revolves around optimizing the "Site Experience" within clinical trials. The panel, featuring representatives from Vanderbilt, aims to provide practical advice and real-world feedback on critical components such as Case Report Forms (CRFs) and the overall usability of the Vault EDC platform. By focusing on the site perspective, the session addresses the significant industry challenge of reducing administrative burden and improving data quality at the source. This approach underscores the importance of user-centric design in regulated enterprise software, ensuring that systems facilitate, rather than hinder, the rapid and accurate execution of clinical protocols. The discussion is structured to cover the entire spectrum of study management, from initial startup procedures through ongoing study execution. This comprehensive scope suggests that the panelists will delve into how Vault EDC streamlines the often-laborious process of site initiation, data entry, query resolution, and overall study management. For technology providers and consultants in the life sciences space, understanding these site-level pain points—particularly concerning CRFs and EDC usability—is essential for developing effective integration strategies and AI-powered tools that enhance clinical operations efficiency and compliance. Key Takeaways: • The "Site Experience" is a primary focus area for technology optimization in clinical trials, suggesting that adoption rates and data quality are highly dependent on the ease-of-use and intuitive design of EDC systems like Veeva Vault EDC. • Direct feedback from major clinical institutions, such as Vanderbilt, offers invaluable, real-world data on the performance and integration challenges associated with regulated software platforms, which is crucial for custom software development and consulting firms. • The panel specifically addresses Case Report Forms (CRFs), highlighting that the design and digital implementation of these forms remain a critical bottleneck or point of optimization for data capture accuracy and site efficiency. • Veeva Vault EDC is positioned as a key solution for simplifying both study startup and ongoing study execution, indicating that the platform offers features designed to accelerate site activation and streamline data management workflows. • The emphasis on hearing "directly from our clinical sites" signals a growing industry trend toward prioritizing the end-user perspective (nurses, coordinators, investigators) when evaluating and implementing clinical technology, moving away from purely sponsor-centric solutions. • For firms specializing in data engineering and business intelligence, the insights gathered from site experience panels can inform the design of robust data pipelines that account for common data entry errors or workflow inefficiencies identified by users. • The R&D and Quality Summit serves as a vital venue for tracking the evolving landscape of clinical technology, regulatory compliance integration (R&D and Quality often overlap in Veeva Vault), and the adoption of enterprise-grade software within the pharmaceutical sector. • Improving the site experience through technology directly impacts the speed and cost of clinical trials; therefore, solutions that leverage AI or automation to further simplify data entry or query management within the EDC environment are highly valuable. Tools/Resources Mentioned: * Veeva Vault EDC (Electronic Data Capture) * Veeva R&D and Quality Summit Key Concepts: * **Site Experience:** Refers to the overall ease-of-use, efficiency, and satisfaction clinical site personnel (coordinators, investigators) have when interacting with the technology and processes required to conduct a clinical trial. Optimizing this experience is key to reducing site burden and improving data quality. * **CRFs (Case Report Forms):** Standardized documents or electronic forms used to collect data on each trial participant as required by the study protocol. The design and usability of CRFs are fundamental to accurate data capture. * **Vault EDC:** Veeva's cloud-based Electronic Data Capture system, used by pharmaceutical companies and CROs to manage clinical trial data collection and cleaning.

Kyle Boelter | Demo: Vault Training - Register for Veeva Summit on October 14
Veeva Systems Inc
/@VeevaSystems
Oct 4, 2021
This video provides a promotional overview of a session focusing on the implementation and value of the Vault Unified Training application, presented at the Veeva R&D and Quality Summit. The central theme is the optimization of personnel training and qualification processes within regulated life sciences enterprises using Veeva's integrated platform. The session, led by Kyle Boelter, aims to demonstrate how this unified application enhances operational efficiency and ensures regulatory compliance across the organization. The demonstration is structured around two key functional improvements designed to streamline the training lifecycle. The first major area of focus is achieving "speed of qualification" for learners through the use of automated assignments. In the pharmaceutical and biotech sectors, rapid and accurate qualification is essential for roles governed by GxP (Good Practices) regulations, clinical trial protocols, and manufacturing standards. By automating the assignment of required training based on role, location, or status, the system minimizes administrative overhead and accelerates the time it takes for personnel to become compliant and productive. This automation capability is critical for maintaining operational continuity and audit readiness. The second core feature highlighted is the introduction of the "new learner home page." This interface is positioned as a significant improvement to the user experience, functioning as a "one-stop page" for all training-related activities. Learners can utilize this centralized hub to view their overall training status instantly and complete all assigned modules without navigating disparate systems. This unified approach reduces user friction, improves training adherence rates, and ensures that comprehensive, verifiable records of training completion are generated, which is paramount for satisfying strict regulatory requirements such as 21 CFR Part 11 and GxP documentation standards. The overall message emphasizes leveraging the unified Veeva Vault platform to achieve both efficiency gains and robust compliance management in R&D and Quality operations. Key Takeaways: * **Consolidation of GxP Training on Vault:** The focus on "Vault Unified Training" confirms Veeva’s strategy to integrate essential GxP functions (Quality, R&D, and Training Management) onto a single platform, creating a unified data environment for compliance and operational reporting. * **Automation as a Compliance Accelerator:** The emphasis on "automated assignments" highlights the industry shift toward intelligent, rule-based training delivery. This automation is crucial for reducing manual errors in assigning complex GxP curricula and ensuring rapid compliance for new hires or personnel changing roles. * **Data Engineering Opportunity in Training Metrics:** A unified training platform generates rich data on qualification speed, completion rates, and compliance status. IntuitionLabs can leverage its data engineering expertise to build robust data pipelines integrating this Vault Training data with other enterprise systems (e.g., HR, Clinical Operations) to create holistic compliance and business intelligence dashboards. * **Optimizing the Learner Experience for Adherence:** The "new learner home page" addresses a common pain point in regulated training: fragmented systems leading to confusion and delayed completion. Improving the user interface directly translates to higher training adherence and better audit readiness. * **AI Enhancement for Training Operations:** The automation mentioned provides a foundation for advanced AI/LLM solutions. Generative AI agents could be developed to act as a "Sales Ops Assistant" or "Medical Info Chatbot" for training, helping learners quickly find specific SOP sections, answer policy questions, or personalize learning paths based on performance data. * **Regulatory Impact of Qualification Speed:** Achieving "speed of qualification" is a direct measure of operational efficiency and regulatory risk mitigation. Faster, verifiable qualification minimizes the time personnel operate without required training, reducing the likelihood of compliance violations during audits. * **Targeting R&D and Quality Operations:** The session's context at the R&D and Quality Summit confirms that training management is a top-tier priority for these departments, which handle highly sensitive data and processes (e.g., clinical trial protocols, manufacturing quality control). * **Enterprise Integration Requirements:** Deploying a unified training application "across your enterprise" necessitates specialized consulting for system integration, data migration, and organizational change management, aligning perfectly with expertise in large-scale Veeva deployments and customization. * **Audit Trail Simplification:** A unified platform inherently simplifies the audit process by centralizing training records, status, and completion verification, helping companies adhere to stringent documentation requirements like 21 CFR Part 11. Tools/Resources Mentioned: * Veeva Vault Training (Vault Unified Training application) Key Concepts: * **Vault Unified Training:** A specific application within the Veeva Vault suite designed to manage all enterprise training requirements, consolidating training curricula, assignments, and records onto a single, regulated platform. * **Automated Assignments:** A feature that automatically assigns required training modules to personnel based on predefined rules, such as their job role, location, or previous qualifications, thereby accelerating the qualification process. * **Speed of Qualification:** A key performance indicator (KPI) measuring the efficiency and rapidity with which personnel complete necessary training and become qualified for their regulated tasks. * **Learner Home Page:** A centralized, "one-stop" user interface designed to provide trainees with an immediate overview of their training status and easy access to all assigned modules, improving user experience and completion rates.

Patient Trust in Healthcare: 84% Trust Doctors, 33% Health Insurance Companies
AHealthcareZ - Healthcare Finance Explained
@ahealthcarez
Oct 3, 2021
This video provides an in-depth exploration of patient and doctor trust within the healthcare system, drawing insights from a January 2021 survey conducted by the American Board of Internal Medicine (ABIM). Dr. Eric Bricker, the presenter, uses the survey data to highlight significant disparities in trust levels across various healthcare entities, including nurses, doctors, government, pharmaceutical companies, health insurance companies, and hospitals. The core purpose of the video is to argue that understanding these trust dynamics is fundamental for driving any meaningful change in healthcare, as trust (ethos) is the prerequisite for persuasion and action. The presentation details patient trust levels, revealing that nurses and doctors command the highest levels of confidence, with 85% and 84% trust respectively. Hospitals also receive a relatively high trust rating from patients at 72%. However, trust significantly diminishes when it comes to the government (56%), pharmaceutical companies (34%), and health insurance companies (33%). This stark contrast underscores a foundational challenge for sectors like pharmaceuticals and health insurance in engaging with the patient population. Dr. Bricker then shifts to doctor trust, presenting even more striking figures. While doctors overwhelmingly trust other doctors (94% within their practice, 85% outside), their trust in hospital executives is surprisingly low at 53%. More critically for the pharmaceutical industry, only 47% of doctors trust pharmaceutical companies, a figure lower than the general public's trust in the government regarding health matters. Trust in health insurance companies plummets to an abysmal 19% among doctors. Dr. Bricker emphasizes that according to the ancient Greek model of persuasion (ethos, pathos, logos), credibility and trust (ethos) must be established first, followed by empathy (pathos), before logic (logos) can be effectively applied. Without this foundational trust, efforts to drive change or influence behavior are largely futile. The video concludes by asserting that entities with low trust, such as health insurance companies, cannot realistically be the drivers of patient or doctor behavior change. Instead, any successful initiative for change must originate from or be channeled through highly trusted sources, primarily nurses and doctors for patients, and other doctors for physicians. This perspective offers a critical lens through which to view strategies for commercial operations, patient engagement, and professional education within the pharmaceutical and life sciences sectors, highlighting the imperative of building or leveraging existing trust. Key Takeaways: * **High Trust in Healthcare Professionals:** Patients exhibit very high levels of trust in nurses (85%) and doctors (84%), positioning these professionals as the most credible sources within the healthcare ecosystem. * **Low Patient Trust in Key Industry Players:** Patient trust in pharmaceutical companies (34%) and health insurance companies (33%) is remarkably low, indicating a significant hurdle for these sectors in direct patient engagement and communication. * **Doctor Trust Dynamics:** Doctors place extremely high trust in their peers (94% for those in their practice, 85% for those outside), suggesting that peer influence is a powerful mechanism for driving change among physicians. * **Pharmaceutical Industry's Trust Deficit with Doctors:** Only 47% of doctors trust pharmaceutical companies, a critical insight for pharma firms aiming to influence prescribing behavior or introduce new therapies. This low trust necessitates indirect or highly credible communication strategies. * **Extremely Low Doctor Trust in Insurers:** Doctors' trust in health insurance companies is exceptionally low at 19%, making insurers highly ineffective as agents of change for physician behavior. * **Foundational Role of Trust in Persuasion:** Drawing on the ancient Greek model of ethos, pathos, and logos, the video underscores that trust (ethos) is the absolute prerequisite for effective persuasion and driving any form of behavior change. * **Implications for Driving Healthcare Change:** Entities with low trust, such as health insurance companies, are unlikely to be successful drivers of patient or doctor behavior change. Efforts to change behavior must leverage or build upon existing trusted relationships. * **Strategic Communication for Pharma:** Given the low trust in pharmaceutical companies by both patients and doctors, strategies for commercial operations, medical affairs, and patient education must prioritize building credibility or working through highly trusted intermediaries like physicians and nurses. * **Leveraging Trusted Channels:** To influence patient behavior, engaging through nurses and doctors is paramount. To influence doctor behavior, peer-to-peer communication and endorsement from other trusted physicians are likely the most effective approaches. * **Challenge for Hospital Executives:** The low trust of doctors in hospital executives (53%) indicates potential internal friction and challenges in implementing top-down initiatives within hospital systems. * **Data-Driven Understanding of Ecosystem:** The survey data provides a concrete, quantitative understanding of the trust landscape, which is essential for any organization operating in or supporting the pharmaceutical and life sciences industries to design effective solutions and strategies. Tools/Resources Mentioned: * **ABIM Foundation/NORC Survey:** The video references a survey conducted by the American Board of Internal Medicine (ABIM) in January 2021, with data sourced from NORC (National Opinion Research Center at the University of Chicago). The specific source PDF link is provided in the video description: https://www.norc.org/PDFs/ABIM%20Foundation/20210520_NORC_ABIM_Foundation_Trust%20in%20Healthcare_Part%201.pdf Key Concepts: * **Ethos, Pathos, Logos:** An ancient Greek model of persuasion. * **Ethos:** Refers to the speaker's credibility or trustworthiness. The video emphasizes this as the foundational element for persuasion. * **Pathos:** Relates to appealing to the audience's emotions or empathy. * **Logos:** Involves using logic and reason to persuade. The video highlights that logic is only effective after ethos and pathos have been established.

RegTalks about Regulatory Information Management Systems (RIMS)
Asphalion
/@Asphalion.
Oct 1, 2021
This video directly addresses Regulatory Information Management Systems (RIMS) within the pharmaceutical and life sciences industries, a core area for companies seeking to optimize operations and maintain regulatory compliance. **Summary:** The "RegTalks" video, featuring Lidia Canovas from Asphalion and Frank Dickert from EXTEDO, provides an in-depth exploration of Regulatory Information Management Systems (RIMS) and their increasing importance in the pharmaceutical and life sciences sectors. The speakers define RIMS as comprehensive solutions for master product data management, regulatory activity tracking, and integrated document management, serving as a crucial "single source of truth." A central theme is the significant industry transition from xEVMPD to the complex ISO IDMP standard, which necessitates robust RIMS to handle increased data transmission and regulatory compliance. The discussion highlights how RIMS enhance operational efficiency by replacing fragmented manual systems, improve impact assessment capabilities, and are applicable across various regulated fields beyond human medicines, including animal health and medical devices. Furthermore, the video emphasizes that RIMS benefit a wide array of departments beyond regulatory affairs, such as quality, clinical, manufacturing, and supply chain, and stresses the indispensable role of experienced implementation partners in successfully deploying these complex systems. **Key Takeaways:** * **RIMS as a Foundational Compliance System:** Regulatory Information Management Systems (RIMS) are critical enterprise solutions for the life sciences, providing a "single source of truth" for master product data, regulatory activities, and integrated document management, essential for ensuring regulatory compliance and operational efficiency. * **IDMP Transition Drives RIMS Adoption:** The impending transition to the ISO IDMP standard is a major catalyst for RIMS implementation, as it significantly increases data complexity and submission requirements, necessitating sophisticated systems to manage and submit data effectively, often integrated with eCTD submissions. * **Broad Organizational Impact:** RIMS are not exclusive to regulatory affairs but serve a wide range of departments across a life sciences company, including quality, clinical, manufacturing, marketing, and supply chain, by providing centralized access to regulatory information, thereby improving cross-functional decision-making and impact assessment. * **Beyond Human Medicines:** The utility of RIMS extends beyond human pharmaceuticals to other regulated sectors such as animal health, consumer health, and medical devices (e.g., for IVDR/MDR compliance), indicating a broader market need for robust regulatory data management solutions. * **Criticality of Implementation Expertise:** Successful RIMS deployment, especially in the context of IDMP compliance, requires knowledgeable external implementation partners who can provide technical support, guide necessary process changes, and leverage deep industry experience to ensure optimal system configuration and user adoption.

What is Trials Methodology?
HRB-TMRN HRB-TMRN
/@hrb-tmrnhrb-tmrn6411
Oct 1, 2021
This video provides an in-depth exploration of randomized trials and the critical discipline of 'trials methodology.' It begins by establishing the universal importance of good health and healthcare, emphasizing that well-informed decisions are paramount for individuals. The findings of research studies, particularly randomized trials, are presented as an essential ingredient in empowering people to make these health decisions. The video then delves into the core mechanism of randomized trials, explaining how treatments are assigned randomly (often by computer) rather than by medical professionals or participants, to ensure unbiased group formation and reliable results. The presentation meticulously explains the concept of randomization, highlighting its purpose in creating groups with characteristics that are as similar as possible, such as age, gender, and ethnicity. This methodological rigor ensures that any observed differences in outcomes between groups can be confidently attributed to the treatment being tested, rather than to pre-existing variations among participants. The video underscores the profound impact of randomized trials, noting their role in testing new medicines, diagnosing conditions, developing treatments, and ultimately saving lives by identifying the most effective interventions for specific conditions or patient populations. A significant portion of the video is dedicated to introducing 'trials methodology' as a distinct field of study. This discipline focuses on continuously evaluating and improving how clinical trials are planned, conducted, and how their findings are shared. It encourages researchers to question existing practices and seek better ways to execute trials. For instance, trials methodology addresses practical challenges like making participation more relevant and easier for patients and doctors. This involves considering the optimal amount and format of information provided to potential participants, who should invite them, when contact should be made, and what support is necessary for informed decision-making. The video provides a concrete example of trials methodology in action by discussing how researchers are exploring innovative ways to present trial information, such as using video formats instead of traditional written materials, to help patients make more informed choices. Furthermore, trials methodology plays a crucial role in determining the most important 'outcomes' to measure in a trial—those aspects that patients, health professionals, and researchers agree are most relevant for a particular condition. The increasing involvement of patients and the public in trials methodology research is highlighted as a key trend, ensuring that the design and execution of trials are aligned with the needs and perspectives of those directly affected. Key Takeaways: * **Foundation of Informed Healthcare Decisions:** Randomized trials are presented as a cornerstone of evidence-based healthcare, providing essential findings that enable individuals to make well-informed decisions about their health and treatment options. * **Mechanism and Purpose of Randomization:** Randomization involves assigning treatments randomly to participants, typically by computer, to create groups with highly similar characteristics. This crucial step ensures that any observed differences in results are attributable to the treatment itself, not to confounding factors among participants. * **Definition of Trials Methodology:** Trials methodology is an academic discipline focused on systematically studying and improving the processes of planning, conducting, and disseminating the findings of randomized trials, constantly seeking better and more efficient approaches. * **Patient-Centric Trial Design:** A core aspect of trials methodology involves making trial participation more accessible and relevant for patients. This includes optimizing the information provided, the timing of contact, and the support offered to help individuals make informed decisions about joining a trial. * **Optimizing Information Delivery:** Researchers are actively investigating more effective ways to convey trial information to potential participants, such as utilizing video formats over traditional written materials, to enhance comprehension and facilitate informed consent. * **Importance of Relevant Outcome Measures:** Trials methodology helps define and standardize the "outcomes" measured in a trial—the specific aspects of health or disease that are assessed to determine treatment efficacy. These outcomes are ideally agreed upon by patients, health professionals, and researchers to ensure their relevance. * **Patient and Public Involvement:** There is a growing emphasis on involving patients and the public directly in trials methodology research. This collaboration ensures that trial designs and processes are patient-centered and address the real-world concerns and priorities of those affected by medical conditions. * **Impact on Medical Advancement:** Randomized trials are instrumental in testing new medicines and treatments, improving diagnostic methods, and developing strategies to prevent and manage medical conditions, ultimately contributing to saving lives and enhancing public health. * **Continuous Improvement Cycle:** Trials methodology fosters a continuous cycle of evaluation and refinement, prompting researchers to constantly question existing practices and innovate in areas such as participant recruitment, data collection, and result sharing. Key Concepts: * **Randomised Trials:** A type of research study where participants are randomly assigned to different treatment groups (e.g., new medicine vs. placebo or standard care) to objectively assess the effectiveness and safety of interventions. * **Randomisation:** The process of assigning participants to treatment groups by chance, typically using a computer, to minimize bias and ensure that groups are as similar as possible in all characteristics except for the treatment received. * **Trials Methodology:** A field of study dedicated to understanding, evaluating, and improving the methods used to design, conduct, analyze, and report randomized trials, with the goal of enhancing their efficiency, relevance, and reliability. * **Outcomes:** The specific health-related events, measures, or changes that are assessed in a clinical trial to determine the effects of an intervention. These can include symptoms, quality of life, disease progression, or survival rates.
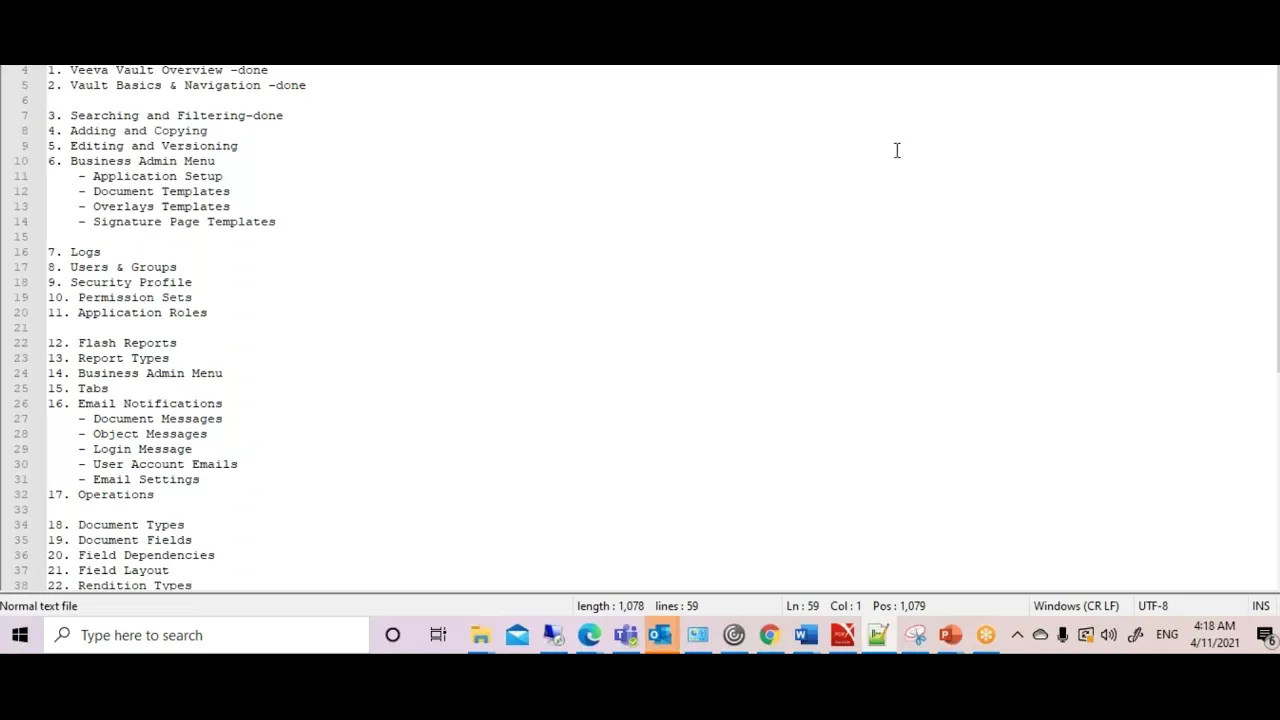
Veeva Vault Training
Albert Jhonson
/@albertjhonson5031
Sep 29, 2021
This video provides a foundational overview of a Veeva Vault training course, outlining the comprehensive curriculum designed to familiarize users with this cloud-based content management solution. The speaker details the various modules and functionalities that will be covered, emphasizing Veeva Vault's superiority in storing documents in a structured manner compared to other cloud solutions. The training is structured to guide participants from basic navigation and user interface understanding to advanced administrative tasks, configurations, and deployment processes, making it suitable for individuals seeking to master the platform for life science industry applications. The course begins with an introduction to the Veeva Vault user interface, including the home page, and then progresses into fundamental operations such as user creation, document creation, editing, and annotation. It covers how documents are displayed within the home directory, leading into the generation of reports and dashboards for data visualization and operational insights. A significant portion of the training is dedicated to the administration section, which delves into business administration, log management, user and group creation, and detailed configurations. This administrative focus ensures that participants understand not only how to use the system but also how to manage and customize it to meet specific organizational needs. Further into the curriculum, the training explores advanced concepts like object and document lifecycles, workflows, and deployment processes. It meticulously breaks down each module, starting with an overview of Veeva Vault, followed by basic navigation and search/filtering techniques. Document operations, including adding, copying, and versioning, are covered in detail, alongside security profiles and mail notifications. The course culminates in an in-depth look at configurations, specifically addressing document types, document fields, and the intricate design of document and object workflows, which are crucial for maintaining regulatory compliance and operational efficiency within the life sciences sector. Key Takeaways: * **Veeva Vault as a Cloud-Based Content Management Solution:** The training positions Veeva Vault as a superior cloud solution for structured document storage and management, highlighting its benefits over generic cloud platforms, particularly for the life sciences industry. * **Comprehensive UI and Navigation Understanding:** Participants will gain a thorough understanding of the Veeva Vault user interface, including the home page, and learn essential navigation techniques to efficiently locate and interact with content. * **Core Document Management Operations:** The course covers critical document operations such as creation, editing, annotation, and versioning, which are fundamental for maintaining accurate and controlled documentation within regulated environments. * **User and Group Administration:** A key focus is placed on user creation, group management, and security profiles, ensuring that administrators can effectively control access and permissions within the Veeva Vault system. * **Reporting and Dashboard Capabilities:** The training includes modules on generating reports and creating dashboards, enabling users to extract actionable insights from their data and monitor key operational metrics. * **In-depth Configuration Management:** The curriculum extensively covers configurations, including the setup of document types, document fields, and the intricate design of document and object lifecycles, which are vital for tailoring the system to specific business processes and regulatory requirements. * **Workflow Automation and Management:** A significant portion of the training is dedicated to understanding and implementing document and object workflows, which are essential for automating processes, ensuring compliance, and streamlining content review and approval cycles. * **Business Administration and Operational Logs:** The course delves into business administration tasks and the management of system logs, providing insights into auditing, troubleshooting, and maintaining the health of the Veeva Vault environment. * **Deployment and System Settings:** Participants will learn about the deployment process for changes within Veeva Vault and how to manage overall system settings, crucial for system maintenance and updates. * **Security and Compliance Focus:** While not explicitly detailed in the transcript, the emphasis on structured content management, user/group security, lifecycles, and workflows inherently supports regulatory compliance (e.g., GxP, 21 CFR Part 11) by ensuring data integrity and auditability. * **Practical Skill Development:** The course is designed to provide practical skills for end-users and administrators alike, moving from basic usage to advanced system configuration and management, enabling efficient utilization of Veeva Vault in a professional setting. **Tools/Resources Mentioned:** * **Veeva Vault:** A cloud-based content management solution specifically designed for the life sciences industry. **Key Concepts:** * **Cloud-Based Solution:** Refers to software and services that run on the Internet instead of locally on your computer, offering scalability, accessibility, and reduced infrastructure overhead. * **Content Management:** The process of organizing, storing, and managing digital content, including documents, images, and other media, throughout its lifecycle. * **Document Lifecycle:** The various stages a document goes through from creation to archiving or deletion, often involving review, approval, and version control. * **Object Lifecycle:** Similar to document lifecycle but applied to specific data objects within the system, defining their states and transitions. * **Workflows:** Automated sequences of tasks or processes that guide content through various stages, ensuring consistency, compliance, and efficiency. * **User Interface (UI):** The visual part of a computer application or website that allows users to interact with it. * **Reports and Dashboards:** Tools for visualizing data and presenting key information in an understandable format, aiding in decision-making and performance monitoring. * **Configurations:** The settings and parameters that define how a software system operates, allowing customization to specific organizational needs.

Employee Demographics - Why Amazon Care Will Fail
AHealthcareZ - Healthcare Finance Explained
@ahealthcarez
Sep 19, 2021
This video provides an in-depth exploration of why employer-sponsored healthcare initiatives, specifically Amazon Care, may struggle to achieve widespread impact due to fundamental differences in employee demographics and associated healthcare costs. Dr. Eric Bricker, the speaker, begins by establishing that the three top diagnostic categories driving employee health plan spend are musculoskeletal conditions (primarily osteoarthritis), cardiovascular diseases (heart attacks, strokes, diabetes), and cancer. He then posits that age is the single greatest risk factor and driver of costs for all these conditions, as bodies naturally deteriorate with age, leading to increased health issues. The speaker supports this claim with specific data from Health Affairs, illustrating a dramatic increase in per-person per-year healthcare costs with age: from $3,628 for under 18s to $18,424 for those 65 and older. He highlights that working populations over 44 years old cost almost double those under 44. This foundational understanding of age as the primary cost driver sets the stage for his critique of Amazon Care. He contrasts the median age of employees in various industries, noting that "older" industries like government (45.6 years), manufacturing (44.4 years), education (43.9 years), and transportation (43.9 years) have median ages significantly above the national average of 42.5 years. Conversely, Dr. Bricker points out that major tech companies like Facebook (28 years), Google (30 years), Apple (31 years), Amazon (31 years), and Microsoft (33 years) have significantly younger workforces. He argues that for these tech giants, employee healthcare is often a "hobby" rather than an "existential threat" because their young, relatively healthy employees incur much lower healthcare costs. Consequently, solutions developed within this context, such as Amazon Care, are designed for a demographic that doesn't face the severe healthcare cost challenges plaguing older industries. The video concludes by suggesting that true innovation and change in employer-sponsored healthcare will emerge from organizations with an "existential threat" – those with older employee populations and consequently higher healthcare burdens, such as state governments, municipalities, school systems, and manufacturing firms. Key Takeaways: * **Age is the Primary Driver of Healthcare Costs:** The video emphatically states that age is the greatest risk factor for the top three diagnostic categories driving employee health plan spend: musculoskeletal, cardiovascular, and cancer. As employees age, their bodies are more prone to these conditions, leading to significantly higher healthcare expenditures. * **Significant Cost Discrepancy by Age Group:** Healthcare costs per person per year escalate dramatically with age. For instance, individuals aged 45-64 cost nearly double those aged 19-44, demonstrating that older workforces inherently incur much higher healthcare expenses. * **Industry Demographics Impact Healthcare Spend:** There's a stark contrast in median employee ages across industries. "Older" industries like government, manufacturing, education, and transportation have median ages significantly above the national average, leading to higher underlying healthcare problems and costs. * **Tech Companies Have a Unique Healthcare Advantage:** Major tech companies (e.g., Facebook, Google, Amazon) boast significantly younger workforces (median ages in the late 20s to early 30s). This demographic advantage means their employees are generally healthier and incur substantially lower healthcare costs. * **Healthcare as a "Hobby" vs. "Existential Threat":** For tech companies with young employees, healthcare costs are often manageable, making it a "hobby" or a perk rather than a critical financial burden. In contrast, for industries with older workforces, healthcare costs can become an "existential threat" to their financial viability. * **Amazon Care's Demographic Mismatch:** Amazon Care, developed for Amazon's young employee base (median age 31), is fundamentally misaligned with the demographics of most other industries facing severe healthcare cost problems. Solutions designed for a young, healthy population are unlikely to be effective or scalable for older, sicker populations. * **Where True Healthcare Innovation Emerges:** Genuine innovation in employer-sponsored healthcare is more likely to originate from organizations facing an "existential threat" from high healthcare costs. These are typically industries with older workforces, such as state and local governments, school systems, and manufacturing companies. * **Examples of Progressive Organizations:** The video cites examples like the State of Montana and the manufacturing company Serigraph as organizations that have successfully implemented changes to address their employee healthcare problems due to their significant "itch" (high costs). * **The "Itch and Scratch" Analogy:** The speaker uses the analogy of an "itch" (poor employee health and high costs) and a "scratch" (healthcare solutions). He argues that tech companies lack a major "itch," thus their "scratches" (solutions) may not be robust enough for those with a severe "itch." * **Technology Adoption Life Cycle in Healthcare:** The video implicitly touches upon the technology adoption life cycle, suggesting that early adopters and pragmatists in industries with high healthcare costs are the ones driving significant change, rather than those without a compelling need. * **Healthcare is Not a Monolith:** Employer-sponsored health plans are highly diverse, primarily due to the varying age and health profiles of employee populations. Solutions must be tailored to these demographic realities, rather than assuming a one-size-fits-all approach. * **Focusing the Mind with Existential Threats:** The speaker emphasizes that "nothing focuses the mind better than an existential threat." This implies that organizations facing severe financial pressure from healthcare costs are more motivated to find and implement truly transformative solutions. Tools/Resources Mentioned: * **Health Affairs:** Cited as a source for healthcare costs by age. * **Bureau of Labor Statistics (BLS):** Cited as a source for employee age by industry. * **Business Insider:** Cited as a source for tech company employee age. * **"16 Lessons in the Business of Healing":** A book by Dr. Bricker, mentioned as a resource for viewers. Key Concepts: * **Employee Demographics:** The statistical characteristics of a workforce, particularly age, which the video highlights as a critical determinant of healthcare costs. * **Healthcare Spend Drivers:** The underlying factors that contribute to the overall cost of healthcare, with age identified as the most significant. * **"Itch and Scratch" Analogy:** A framework used to explain the relationship between a problem (the "itch" of poor health and high costs) and its corresponding solution (the "scratch" of healthcare interventions). * **Technology Adoption Life Cycle:** A sociological model describing the adoption or acceptance of a new product or innovation, applied here to the adoption of healthcare solutions by different types of organizations. Examples/Case Studies: * **Amazon Care:** Used as the central example of a healthcare initiative developed within a demographic context (young tech employees) that the speaker argues makes it unsuitable for broader application. * **State of Montana:** Highlighted as a progressive organization that successfully addressed its state employee health problem, likely due to having an older workforce and thus a significant "itch." * **Serigraph:** A manufacturing company whose CEO wrote a book about solving healthcare, presented as another example of an organization driven by necessity to innovate. * **Cities, Municipalities, and School Systems:** Mentioned as organizations in states like Colorado and Wisconsin that have implemented on-site or near-site clinics due to high healthcare costs, demonstrating localized innovation.

Health Insurance Carrier Earnings Calls: Learn Their TRUE Strategy
AHealthcareZ - Healthcare Finance Explained
@ahealthcarez
Sep 12, 2021
This video provides an in-depth exploration of the true strategic priorities of major health insurance carriers in the United States, as revealed through their Q2 2021 investor earnings calls. Dr. Eric Bricker analyzes the conference calls of industry giants like UnitedHealth Group, CVS Health, Cigna, and Anthem, aiming to uncover what these companies communicate to their investors as their primary drivers of growth and profitability. The analysis highlights a significant divergence between the perceived focus of these carriers and their actual financial strategies, particularly concerning employer-sponsored health plans versus government programs and Pharmacy Benefit Managers (PBMs). The core finding of the video is that the primary growth engines for these large health insurance carriers are government programs, specifically Medicare Advantage and Medicaid managed care, and an increase in script counts for their PBM businesses. Dr. Bricker points out that every single one of these companies emphasized these two areas as their sources of growth. Conversely, there was a striking absence of any mention of employer-sponsored health plans as a growth driver. This creates a disconnect, as the digital health sector, fueled by significant venture capital and private equity investment (e.g., $14 billion in 2020), is heavily focused on creating innovative solutions for employer health plans. The speaker contrasts the market capitalization of digital health innovators like Teledoc with that of established carriers, suggesting missed opportunities for the latter if they were truly focused on innovation in the employer space. Dr. Bricker explains this apparent contradiction by characterizing health insurance carriers as "fast-followers" rather than true innovators. He recounts a personal experience where a large carrier admitted to copying successful solutions from smaller, agile companies. This strategy allows them to offer "just good enough" services to retain employer clients without investing heavily in R&D for this segment. The video further reveals that carriers strategically utilize employer-sponsored plans to negotiate less favorable PPO network discounts with hospital systems. These PPO contracts, while not optimal for employers, enable the carriers to secure much lower HMO allowed amounts, which they then leverage for their more profitable Medicare Advantage and Medicaid managed care members. The speaker underscores the financial motivation, noting that the margin on a Medicare Advantage beneficiary is significantly higher ($1600 per year) than that for an employer-sponsored plan member ($800 per year), making government programs both faster-growing and more profitable. The video concludes by advising employers to listen to the investor calls of their health insurance carriers to understand their genuine priorities. This insight is critical for any entity operating within the broader healthcare ecosystem, including pharmaceutical and life sciences companies, as the strategic decisions of major payers directly influence market access, drug utilization, and commercial success. By understanding where these carriers derive their growth and profit, other industry players can better anticipate market shifts and align their own strategies. Key Takeaways: * **Primary Growth Drivers for Health Insurance Carriers:** Major health insurance carriers like UnitedHealth Group, CVS Health, Cigna, and Anthem primarily derive their growth from government programs (Medicare Advantage and Medicaid managed care) and increased prescription script counts for their integrated PBM businesses (e.g., OptumRx, Caremark, Express Scripts). * **Employer Plans are Not a Growth Priority:** Despite significant market activity, employer-sponsored health plans are explicitly not considered a source of growth by these large carriers in their investor communications. This indicates a strategic de-prioritization of this segment. * **Disconnect in Innovation Investment:** There's a stark contrast between the carriers' lack of focus on employer plans and the substantial venture capital and private equity investment ($14 billion in 2020) pouring into digital health companies specifically creating innovative solutions for employer-sponsored health plans. * **"Fast-Follower" Strategy:** Health insurance carriers tend to be "fast-followers" rather than innovators, preferring to copy successful digital health solutions developed by smaller, agile companies. Their goal is to offer "good enough" services to retain employer clients, not to lead innovation in this space. * **Strategic Use of Employer Plans:** Carriers leverage their employer-sponsored health plans to negotiate PPO network discounts with hospital systems that are "not so hot" for employers. This allows them to secure much lower HMO allowed amounts, which are then utilized for their more profitable Medicare Advantage and Medicaid managed care members. * **Higher Profitability of Government Programs:** Medicare Advantage beneficiaries yield significantly higher profit margins (approximately $1600 per person per year) compared to members on employer-sponsored health plans (approximately $800 per person per year). This financial incentive drives the carriers' strategic focus. * **Implications for Pharmaceutical Commercial Operations:** The emphasis on PBM script counts as a growth driver directly impacts pharmaceutical companies. Understanding this priority is crucial for optimizing market access strategies, drug utilization, and overall commercial success within the pharmaceutical and life sciences sectors. * **Market Access Strategy Context:** For pharmaceutical companies, understanding the payer landscape, particularly the financial drivers of major health insurance carriers and PBMs, is essential for developing effective market access and reimbursement strategies for their products, especially concerning government programs like Medicare Advantage and Medicaid. * **Data-Driven Strategic Insights:** The video highlights the value of analyzing public financial statements and investor calls to uncover the true strategic priorities of key players in the healthcare ecosystem, providing actionable intelligence for other stakeholders. * **Understanding Payer Behavior:** Pharmaceutical and life sciences firms need to recognize that large payers prioritize segments with higher growth and profitability. This understanding should inform how they engage with these payers for product placement, formulary inclusion, and patient access initiatives. Key Concepts: * **Medicare Advantage:** A type of Medicare health plan offered by a private company that contracts with Medicare to provide all your Part A and Part B benefits. * **Medicaid Managed Care:** A system where states contract with managed care organizations (MCOs) to provide healthcare services to Medicaid beneficiaries. * **PBM (Pharmacy Benefit Manager):** A third-party administrator of prescription drug programs for commercial health plans, self-insured employer plans, Medicare Part D plans, the Federal Employees Health Benefits Program, and state government employee plans. * **PPO (Preferred Provider Organization):** A type of health plan where you pay less if you use providers in the plan's network. * **HMO (Health Maintenance Organization):** A type of health insurance plan that usually limits coverage to care from doctors who work for or contract with the HMO. * **Digital Health:** The convergence of digital technologies with health, healthcare, living, and society to enhance the efficiency of healthcare delivery and make medicine more personalized and precise. Examples/Case Studies: * **Health Insurance Carriers:** UnitedHealth Group (with OptumRx PBM), CVS Health (with Caremark PBM), Cigna (with Express Scripts PBM), and Anthem (with its own PBM). * **Digital Health Company:** Teledoc, cited for its significant market capitalization ($22.6 billion) as an example of successful innovation in digital health, contrasting with the carriers' slower adoption.
FDA Pharmaceutical Industry Ties
AHealthcareZ - Healthcare Finance Explained
@ahealthcarez
Sep 6, 2021
This video, presented by Dr. Eric Bricker of AHealthcareZ, delves into the intricate and often problematic financial and personnel ties between the U.S. Food and Drug Administration (FDA) and the pharmaceutical industry it is tasked with regulating. Drawing insights from a 2021 New York Times opinion piece by Farhad Manjoo, Dr. Bricker explores two primary areas of potential conflict of interest: the FDA's funding model and the "revolving door" phenomenon where regulators transition to industry roles. The overarching purpose is to highlight systemic issues that may compromise the FDA's impartiality and, consequently, drug safety for patients, prompting a discussion on how physicians should approach prescribing newly approved medications. The speaker details a significant shift in FDA funding that occurred in 1992, where the agency began collecting fees directly from pharmaceutical companies to cover the salaries of FDA employees. This arrangement, which includes "performance guarantees" from the FDA related to the speed of drug reviews, creates a direct financial link between the regulator and the regulated, raising concerns about "regulatory capture." Dr. Bricker emphasizes that this model means the industry itself funds the very body designed to oversee it, inherently creating grounds for a conflict of interest that could prioritize speed of approval over thoroughness or safety. Further exacerbating these concerns is the "revolving door" phenomenon, where FDA personnel, particularly those involved in drug approval, leave their government positions to take up more lucrative roles within the pharmaceutical industry. The video cites an example of over 13 cancer drug reviewers making this transition. A particularly egregious case highlighted is that of Curtis Wright, the FDA regulator specifically responsible for Oxycontin, who subsequently left the FDA to work for Purdue Pharma, the manufacturer of Oxycontin and a central figure in the opioid epidemic. These instances suggest a potential for regulators to be overly lenient in the approval process, possibly with an eye toward future employment opportunities within the industry. The consequences of these ties are illustrated by a troubling statistic: one-third of drugs approved by the FDA between 2000 and 2010 were later found to have safety problems during Phase 4 post-market surveillance. In response to these systemic issues, Dr. Bricker shares a practical recommendation from his residency training at Johns Hopkins: physicians might consider waiting five years after a new drug's approval before prescribing it, especially if other proven and safe alternatives exist. This allows for more extensive real-world data collection and identification of issues missed in initial clinical trials, as seen with drugs like Vioxx, Avandia, and Zelnorm. While acknowledging exceptions for critical medications with no alternatives, such as the COVID vaccine, the video concludes with a strong call for fundamental changes to the FDA's funding and operational structure to restore public trust and ensure unbiased regulation. Key Takeaways: * **FDA Funding Model Conflict:** Since 1992, the FDA has received funding directly from pharmaceutical companies to pay employee salaries, creating a direct financial tie and potential conflict of interest, as the regulated industry funds its regulator. * **Performance Guarantees:** In exchange for industry funding, the FDA reportedly offered "performance guarantees" related to the speed of drug reviews, suggesting a prioritization of efficiency that could compromise thoroughness. * **High Incidence of Post-Approval Safety Issues:** A significant concern is that one-third of drugs approved by the FDA between 2000 and 2010 were later found to have safety problems during Phase 4 post-market surveillance, indicating potential gaps in the initial approval process. * **The "Revolving Door" Phenomenon:** Many FDA regulators transition to high-paying jobs within the pharmaceutical industry after their tenure, raising ethical questions about potential leniency during their regulatory roles. * **Egregious Example of Conflict:** The case of Curtis Wright, the FDA regulator for Oxycontin, who later worked for Purdue Pharma, serves as a stark illustration of how the "revolving door" can lead to severe conflicts of interest with public health consequences. * **Physician's Prudent Prescribing Strategy:** A recommendation from Johns Hopkins suggests physicians consider waiting five years after a new drug's approval before prescribing it, allowing for more robust post-market safety data to emerge. * **Importance of Phase 4 Surveillance:** This waiting period emphasizes the critical role of Phase 4 post-market surveillance in identifying adverse effects or safety issues that may not be apparent during pre-market clinical trials. * **Historical Precedents for Caution:** Past examples like Vioxx (increased cardiovascular risk), Avandia (poor cardiovascular outcomes), and Zelnorm (liver function problems) underscore the historical basis for exercising caution with newly approved medications. * **Exceptions for Unmet Needs:** The strategy of waiting five years is not absolute; in situations where no other effective treatment options exist (e.g., the COVID vaccine), the immediate use of new drugs may be justified, balancing risks and benefits. * **Broader Regulatory Context:** When evaluating critical new drugs, especially in global health crises, considering approvals by multiple international regulatory agencies can provide an additional layer of validation beyond the FDA's assessment. * **Call for Systemic Reform:** The video advocates for fundamental changes to the FDA's funding and operational structure to eliminate conflicts of interest, enhance regulatory independence, and rebuild public trust in drug approval processes. Tools/Resources Mentioned: * New York Times article by Farhad Manjoo (September 2, 2021) * AHealthcareZ video on Regulatory Capture * AHealthcareZ video on Stages of Drug Approval Key Concepts: * **Regulatory Capture:** A form of political corruption that occurs when a regulatory agency, created to act in the public interest, instead advances the commercial or political concerns of special interest groups that dominate the industry or sector it is charged with regulating. * **Post-Market Surveillance (Phase 4):** The ongoing monitoring of a drug's safety and effectiveness after it has been released to the market and is being used by the general public. This phase is crucial for detecting rare or long-term side effects that may not have been observed during clinical trials. * **Conflict of Interest:** A situation in which a person or organization has a vested interest—financial, personal, or otherwise—that could potentially bias their judgment or actions in a professional or official capacity. * **Quid Pro Quo:** A favor or advantage granted or expected in return for something. In this context, it refers to the implied exchange of industry funding for faster drug review times or future job opportunities. Examples/Case Studies: * **Curtis Wright and Oxycontin:** The FDA regulator responsible for Oxycontin who later took a job with Purdue Pharma, the drug's manufacturer, illustrating a direct "revolving door" conflict. * **Vioxx:** A pain medication found to increase cardiovascular and stroke risk after its approval. * **Avandia:** A diabetes medication found to increase poor cardiovascular outcomes post-approval. * **Zelnorm:** An IBS medication taken off the market due to problems with liver function. * **COVID Vaccine:** Cited as an example of a critical new drug where immediate use was justified due to the lack of alternatives and global pandemic circumstances, despite broader concerns about FDA processes.

portatour® route planner for Veeva CRM – demo video
portatour®
/@portatour
Sep 1, 2021
This video provides an in-depth exploration of portatour®, an artificial intelligence route planning system designed to integrate seamlessly with Veeva CRM for pharmaceutical field representatives. The primary purpose of the video is to demonstrate how this solution helps reps achieve their cycle plan goals, optimize their schedules, minimize driving time, and ultimately increase customer visits. It highlights the inefficiencies of manual planning and positions portatour as a user-friendly, automated solution that leverages AI to enhance commercial operations within the life sciences sector. The video begins by outlining the common challenges faced by field reps, such as Robert Rep, who struggle to manually plan visits to Healthcare Professionals (HCPs) while adhering to cycle plan goals, prioritizing accounts, and minimizing driving distances. It illustrates how traditional planning methods are time-consuming and often result in missed targets despite significant effort. Portatour addresses this by reading critical data directly from Veeva CRM, including customer addresses, cycle plan targets, achieved calls, last call dates, remaining calls, saved appointments, and office best times. An artificial intelligence algorithm then analyzes this information to select optimal customers for each day, considering geographical proximity and historical traffic data, to generate highly efficient routes. The core output of portatour is the "magic calendar," where these optimized call suggestions are automatically written back into the Veeva CRM calendar. This feature intelligently distributes remaining calls throughout the cycle plan, taking into account existing appointments and filling any gaps, ensuring reps have a complete and optimized schedule. The solution maintains a user-friendly experience by allowing reps to continue working within their familiar Veeva CRM mobile environment. Furthermore, the video introduces "portatour anywhere," a web-based interface that provides reps with a visual map of their territory, color-coding customers based on visit urgency (green for recently visited, yellow for due, red for overdue), allowing for quick strategic oversight and route adjustments. The video concludes by detailing the simple deployment process for portatour within an existing Veeva CRM environment. This involves an initial analysis of the Veeva installation, followed by installation as a managed solution and collaborative configuration based on the client's business strategy. A pilot project with a limited number of reps is recommended for initial rollout. The pricing model is presented as Software-as-a-Service (SaaS), with ongoing costs per user per month and one-time costs for the base license and rollout project. The video emphasizes a clear return on investment, stating that portatour pays off by optimizing mileage by more than two percent or achieving two additional calls per month, with expected performance increases of up to 25%. Key Takeaways: * **AI-Powered Route Optimization:** Portatour is an artificial intelligence system specifically designed to automate and optimize route planning for pharmaceutical field reps using Veeva CRM, significantly reducing manual planning effort. * **Seamless Veeva CRM Integration:** The system directly reads essential data from Veeva CRM, such as customer addresses, cycle plan targets, call history, and existing appointments, and writes optimized schedules back into the Veeva CRM calendar, ensuring a cohesive workflow. * **Enhanced Cycle Plan Achievement:** By automatically prioritizing and scheduling calls based on cycle plan goals and customer urgency, portatour helps reps consistently meet their targets and visit more customers. * **Minimized Driving Time and Increased Efficiency:** The AI algorithm considers geographical proximity and historical traffic data to create the most efficient routes, minimizing driving time and enabling reps to conduct more calls per day. * **"Magic Calendar" for Automated Schedules:** Reps receive a pre-populated, optimized weekly schedule directly within their Veeva CRM calendar, which intelligently distributes remaining calls and fills gaps, simplifying their daily planning. * **User-Friendly Experience:** Field reps continue to operate within their familiar Veeva CRM mobile interface, making the portatour connector a non-disruptive and intuitive solution. * **Visual Territory Management:** The "portatour anywhere" web application provides a visual map of the rep's territory, color-coding customers by visit urgency (green, yellow, red), allowing for quick strategic overview and identification of high-priority visits. * **Strategic Deployment Process:** Rolling out portatour involves an initial analysis of the existing Veeva CRM installation, installation as a managed solution, collaborative configuration, and a recommended pilot project for smooth adoption. * **Clear Return on Investment (ROI):** The solution is presented with tangible ROI metrics, indicating it pays off by optimizing mileage by over two percent or enabling two additional calls per rep per month, with potential performance increases of up to 25%. * **SaaS Pricing Model:** Portatour is offered as a Software-as-a-Service, including all updates and support, with ongoing per-user monthly costs and one-time costs for the base license and the initial rollout project. * **Free Admin/Supervisor Accounts:** A notable benefit is that administrative and supervisor accounts are free, reducing overall licensing costs for the organization. * **Focus on Pharma Field Reps:** The solution is specifically tailored for the unique needs of pharmaceutical field representatives, addressing challenges related to HCP visits and cycle plan management in a regulated environment. Tools/Resources Mentioned: * Veeva CRM * portatour® (AI route planning system) * portatour® anywhere (web-based map interface) Key Concepts: * **Cycle Plan:** A strategic plan in Veeva CRM defining how often specific Healthcare Professionals (HCPs) need to be visited based on priority and potential. * **AI Algorithm:** The core intelligence of portatour that analyzes various data points to optimize call schedules and routes. * **Geographic Optimization:** The process of planning routes to minimize driving distances and time between customer visits. * **Managed Solution:** A deployable package in Veeva CRM that allows for easy installation and management of the portatour connector. * **SaaS (Software as a Service):** A software licensing and delivery model in which software is licensed on a subscription basis and is centrally hosted.

Vault CTMS Demo: Risk-based Study Management
Veeva Systems Inc
/@VeevaSystems
Sep 1, 2021
This video provides an in-depth demonstration of Veeva Vault CTMS’s capabilities for Risk-Based Study Management (RBSM), illustrating a unified approach to optimizing clinical trial operations. The core purpose of the tool is to enable life sciences organizations to seamlessly identify, assess, remediate, and monitor risks across individual clinical trials and analyze trends seen across an entire study portfolio. By centralizing risk management processes, the system aims to improve data quality, reduce operational risk, and ensure resources are allocated most effectively to high-risk areas. The process begins with the establishment of a centralized location for storing risk templates. This library can include pre-defined risk mitigation action items and categories, which form the baseline for risk assessment templates used at the study level. Multiple assessment templates can be incorporated, such as a standard Risk Assessment Template (RAFT), allowing users to pull in numerous risks from the library. When drilling into a specific risk, the system allows for setting default values, assigning examples, and defining mitigation items. Crucially, the platform provides an area to view how that specific risk has been assessed across all multiple studies that have utilized it, driving standardization and learning across the organization. Once approved templates are established, generating a study-specific assessment is streamlined. Users can select one or multiple approved templates as a starting point, or alternatively, leverage an existing study’s completed risk assessment if the new trial is structurally similar. Within the newly generated assessment, users can easily adjust the three core risk factors—impact, probability, and detectability—to auto-calculate a precise risk score. This score is accompanied by a visual icon, making the level of risk associated with each line item immediately clear. Mitigation action items can be selected, and these actions are set to automatically generate and send out tasks to the appropriate roles listed, such as Clinical Research Associates (CRAs). Following the completion of the assessment, it is submitted through an out-of-the-box workflow for review and approval by designated groups or individuals. Upon approval, the system automatically generates a comprehensive assessment document inclusive of all data and files it directly to the Trial Master File (TMF) side of Vault Clinical, eliminating manual filing effort. Subsequently, all mitigation action items are automatically dispatched to the relevant users' task queues. CRAs, for instance, receive site-level tasks detailing the risk, linking back to the assessment, and providing an area to track resolution details, including dates captured in the background to measure cycle times. All this collected data culminates in centralized dashboards, which provide actionable business intelligence by visualizing trends around the most difficult-to-detect, highest-impact, and highest-probability risks across the entire portfolio, allowing organizations to compare study scores and identify the riskiest trials for immediate intervention. Key Takeaways: • **Centralized Risk Library:** Vault CTMS provides a single source of truth for storing risk templates, mitigation action items, and categories, ensuring consistent application of risk management standards across all clinical trials. • **Flexible Assessment Generation:** Studies can initiate risk assessments either by selecting from multiple approved, standardized templates (e.g., a standard RAFT) or by cloning a completed risk assessment from a structurally similar existing study, promoting efficiency. • **Automated Risk Scoring:** Risk scores are dynamically calculated based on user inputs for Impact, Probability, and Detectability, providing immediate visual feedback (via icons) on the severity level of each identified risk. • **Workflow-Driven Approval:** The system utilizes an out-of-the-box workflow for assessment review and approval, allowing users to select single or group approvers, ensuring necessary oversight before implementation. • **Seamless TMF Integration:** Upon final approval of the risk assessment, the system automatically generates the assessment document and files it directly into the TMF within Vault Clinical, ensuring compliance and eliminating manual documentation steps. • **Automated Task Assignment:** Selecting mitigation action items during the assessment phase automatically triggers the generation and distribution of tasks to the appropriate roles (e.g., CRAs), streamlining the remediation process. • **Site-Level Task Management:** Assigned users, such as CRAs, receive specific site-level tasks in their queue, complete with details about the risk, links to the assessment, and a dedicated area to track resolution status and completion dates. • **Cycle Time Tracking:** The platform tracks resolution dates in the background, capturing cycle times for risk mitigation activities, which provides valuable metrics for optimizing operational efficiency and identifying bottlenecks. • **Portfolio-Level Business Intelligence:** Comprehensive dashboards aggregate risk data across all studies, allowing management to view trends related to the highest impact, highest probability, and most difficult-to-detect risks across the entire portfolio. • **Comparative Study Analysis:** The dashboards enable users to compare risk scores between different studies, facilitating the identification of the riskiest trials that require immediate, targeted resource allocation and intervention. • **Standardization and Learning:** The ability to view how a specific risk has been assessed across multiple studies allows organizations to continuously refine their risk definitions, mitigation strategies, and templates based on real-world outcomes. Tools/Resources Mentioned: * Vault CTMS (Clinical Trial Management System) * Vault Clinical * TMF (Trial Master File) Key Concepts: * **Risk-Based Study Management (RBSM):** A methodology focused on proactively identifying, assessing, and controlling risks that could affect the quality and safety of clinical trial data and subjects. * **Risk Assessment Template (RAFT):** A standardized document or digital template used to categorize and quantify potential risks within a clinical trial, often serving as a starting point for study-specific assessments. * **Impact, Probability, and Detectability:** The three core factors used in the system to calculate a quantitative risk score, determining the severity and likelihood of a risk event occurring and the ease with which it can be identified.

Veeva Vault RIM
Veeva Systems Inc
/@VeevaSystems
Aug 31, 2021
This video provides an in-depth exploration of the challenges inherent in traditional Regulatory Information Management (RIM) within the life sciences industry and introduces the Veeva Vault RIM suite as a unified solution to these complexities. The presentation begins by establishing that managing regulatory information is incredibly difficult, particularly when supporting multiple global markets. It highlights how routine events, such as a manufacturing change or a health authority request, trigger extensive, necessary activities—including impact assessments, submission updates, and ensuring regional compliance—all of which are complicated by fragmented, disjointed regulatory systems. The video details the typical, inefficient process for managing a product-related change. This process necessitates conducting regulatory impact assessments by checking headquarters' registration tracking systems, requesting information from global affiliates via calls or emails, and then aggregating this data into a separate project tracker. Subsequent steps involve managing submission authoring and reviews in one system, exporting content to another system for publishing, dispatching the submission, updating multiple trackers with health authority responses, and finally storing the completed dossier in a separate repository or file share. The core problem identified is that with planning, execution, and tracking residing in separate, non-integrated systems, compliance becomes challenging and achieving end-to-end visibility is nearly impossible. Veeva Vault RIM is presented as the transformative solution that unifies regulatory documents and data onto a single, cloud-based platform. This consolidation is designed to eliminate information silos and streamline regulatory processes globally. By moving to this unified system, regulatory teams gain access to real-time tracking for all submission documents and regulatory activities across every affected market, ensuring consistency and accelerating workflows. The Vault RIM suite is composed of several integrated applications, each addressing a critical component of the regulatory lifecycle. Vault Registrations manages global product registration data; Vault Submissions streamlines the entire process from planning to collaborative authoring and final approval; Vault Submissions Publishing automatically validates and publishes dossiers for distribution to health authorities; and Vault Submissions Archive securely stores and provides viewing access to the complete history of submissions in the cloud. This integrated approach ensures that organizations have one system providing comprehensive insight into global regulatory information and activities, enabling them to move faster, improve visibility, and strengthen overall compliance. Key Takeaways: • **Fragmentation Drives Compliance Risk:** The traditional regulatory process is characterized by the use of multiple, separate systems for tracking, authoring, publishing, and archiving. This disjointed nature makes maintaining compliance difficult and prevents end-to-end visibility into global regulatory status. • **Complexity of Routine Events:** Even common occurrences like a manufacturing change or a health authority request trigger complex, multi-step regulatory workflows that require coordination across headquarters and affiliates, often relying on manual follow-up via calls and emails. • **Unification as a Core Value Proposition:** Veeva Vault RIM’s primary benefit is eliminating information silos by unifying all regulatory documents and data onto a single, cloud-based platform, thereby simplifying complex global processes. • **Real-Time Global Activity Tracking:** The platform provides regulatory teams with the ability to track submission documents and regulatory activities in real-time across all affected global markets, which is crucial for managing international compliance obligations. • **Dedicated Registration Management:** Vault Registrations serves as the centralized system for managing all global product registration data, ensuring a single source of truth regarding product status and regulatory requirements worldwide. • **Streamlining Submission Workflows:** Vault Submissions integrates planning, collaborative authoring, and approval processes, replacing the need for separate project trackers and review systems and accelerating the time required to prepare regulatory filings. • **Automated Publishing and Validation:** Vault Submissions Publishing automates the technical aspects of dossier preparation, including validation, ensuring that submissions are compliant with health authority specifications prior to distribution. • **Secure, Integrated Archiving:** Vault Submissions Archive provides secure, cloud-based storage for the complete submission history (the final dossier), ensuring long-term accessibility and adherence to regulatory requirements for records management. • **Integrated IDMP Readiness:** The Veeva Vault RIM suite is noted to include fully integrated capabilities for IDMP (Identification of Medicinal Products), positioning companies to meet this evolving global regulatory standard. • **Improved Business Outcomes:** The shift to a unified system provides organizations with the necessary insight to improve visibility, accelerate regulatory timelines, and strengthen their overall compliance posture. Tools/Resources Mentioned: * Veeva Vault RIM Suite * Vault Registrations * Vault Submissions * Vault Submissions Publishing * Vault Submissions Archive Key Concepts: * **Regulatory Information Management (RIM):** The systematic management of all regulatory data, documents, and processes required to bring and keep pharmaceutical products on the market globally. * **IDMP (Identification of Medicinal Products):** A set of five ISO standards defining the internationally agreed-upon data elements and structures for the unique identification of medicinal products. * **Submission Archiving:** The secure, compliant storage and management of completed regulatory dossiers and submission histories, often required for audit trails and long-term regulatory reference.

GE Healthcare: Jeff Immelt's Autobiography Reveals Why Zero Growth
AHealthcareZ - Healthcare Finance Explained
@ahealthcarez
Aug 29, 2021
This video provides an in-depth exploration of GE Healthcare's stagnant growth over a decade, using insights from former GE CEO Jeff Immelt's autobiography, "Hot Seat." Dr. Eric Bricker, the speaker, uses GE Healthcare as a case study to illustrate how established business models can become significant barriers to innovation and adaptation within the broader healthcare industry. The analysis delves into the underlying economic drivers that prevent large organizations from embracing new technologies and market shifts, ultimately leading to missed opportunities and underperformance compared to more agile competitors. The core of the video's argument centers on GE Healthcare's business model, which relies on selling medical devices like CT scanners, MRI machines, and ventilators at cost, and then generating the vast majority of its profit from subsequent service contracts for maintenance and repairs. This model, while historically profitable, creates a perverse incentive: improving product efficiency or durability directly reduces the lucrative service revenue. Immelt's attempt to counter this with "GE Digital," an initiative aimed at leveraging the Internet of Things (IoT) to make GE devices more efficient and easier to service, was met with internal resistance and eventually abandoned because it threatened the existing revenue streams. Dr. Bricker extends this specific example to a broader critique of large, bureaucratic organizations within healthcare, including hospitals and Pharmacy Benefit Managers (PBMs). He argues that many are "married to their old business models"—such as fee-for-service in hospitals or commission-based structures in PBMs—making it incredibly difficult for them to pivot towards patient-centered care, prevention, or other innovative approaches. The video contrasts GE Healthcare's stagnation with the rapid growth of newer, technology-focused companies like Health Catalyst (software analytics), Teledoc (virtual visits), and Veeva Solutions (cloud computing for biopharma), highlighting how GE missed opportunities to acquire or develop similar capabilities, leading to a significant loss of market capitalization and relevance in emerging healthcare sectors. Key Takeaways: * **Business Model as an Innovation Barrier:** GE Healthcare's reliance on service contracts for maintenance and repairs created a disincentive for developing more durable or efficient medical devices. This illustrates how an established revenue model can actively hinder product innovation and digital transformation efforts. * **Organizational Resistance to Change:** Jeff Immelt's "GE Digital" initiative, aimed at improving product efficiency through IoT, failed due to internal organizational revolt. This highlights the profound challenge large, established companies face when attempting to disrupt their own profitable, albeit outdated, business practices. * **Economic Models Drive Behavior:** The video emphasizes that the underlying economic business model of an organization ultimately dictates its actions and priorities. In healthcare, this means that despite discussions of patient-centeredness or prevention, the fee-for-service model often ensures that revenue generation from procedures and treatments prevails. * **Stagnant Growth in Established Giants:** GE Healthcare experienced near-zero growth (0.69% CAGR) over a decade, significantly underperforming competitors like Medtronic (7% CAGR). This demonstrates the severe consequences of failing to adapt and innovate in a dynamic industry. * **Missed Opportunities in Emerging Technologies:** GE Healthcare failed to capitalize on the rise of new healthcare technology companies. It missed opportunities to acquire or develop capabilities in areas like software analytics (Health Catalyst), virtual care (Teledoc), and cloud computing for biopharma (Veeva Solutions), all of which achieved substantial market capitalization during GE's stagnant period. * **The Rise of Agile Innovators:** Companies like Health Catalyst ($2.7B market cap), Teledoc ($22.8B market cap), and Veeva Solutions ($50B market cap) emerged and thrived by focusing on software, data, and cloud-based solutions, demonstrating the market's demand for innovative, technology-driven healthcare services. * **Veeva Solutions as a Benchmark:** The explicit mention of Veeva Solutions, founded in 2007 and achieving a $50 billion market capitalization by 2021 through cloud computing for the biopharma industry, underscores the immense value created by specialized, digitally native solutions in the life sciences sector. * **Challenges for Large Bureaucratic Organizations:** The video concludes that large, bureaucratic organizations often struggle to break from their past and adapt to new market realities, even when leadership recognizes the need for change. This suggests that innovation is more likely to come from outside these established structures. * **Implications for Career Choices:** For individuals aspiring to drive significant change in healthcare, the speaker advises against working for large, established healthcare companies, suggesting that their inherent resistance to change makes impactful innovation difficult from within. * **The Power of Data and Analytics:** The success of Health Catalyst highlights the growing importance of software analytics for hospital systems, a domain where traditional hardware manufacturers like GE Healthcare failed to establish a strong presence. * **Transparency from Retired Leaders:** Jeff Immelt's autobiography is presented as a valuable source of candid insights, as retired leaders are often more forthright about organizational challenges and failures than when they are actively in their roles. **Tools/Resources Mentioned:** * **"Hot Seat" by Jeff Immelt:** Autobiography of the former CEO of GE. * **Bloomberg.com:** Financial news and data source. * **Veeva.com:** Website for Veeva Solutions. * **Macrotrends.net:** Financial data website (used for Medtronic revenue). * **Statista.com:** Statistics portal (used for GE Healthcare revenue). **Key Concepts:** * **Compound Annual Growth Rate (CAGR):** A measure of the annual growth rate of an investment over a specified period longer than one year. * **Planned Obsolescence:** The practice of designing products to have a limited lifespan, often to encourage repeat purchases or service contracts. In this context, it refers to the disincentive to create highly durable products. * **Fee-for-Service:** A payment model in healthcare where services are unbundled and paid for separately, often criticized for incentivizing volume over value or prevention. * **Internet of Things (IoT):** A network of physical objects embedded with sensors, software, and other technologies for the purpose of connecting and exchanging data with other devices and systems over the internet. * **P/E Ratio (Price-to-Earnings Ratio):** A valuation ratio of a company's current share price compared to its per-share earnings. **Examples/Case Studies:** * **GE Healthcare's Stagnation:** Revenue growth of only 0.69% CAGR from $16.9B (2010) to $18.1B (2020), compared to Medtronic's 7% CAGR during the same period. * **GE Capital's Unwinding:** The financial crisis of 2008-2009 decimated GE Capital, a banking division that constituted half of GE's revenue, leading to its eventual divestment. * **Failure of GE Digital:** An initiative to improve the efficiency of GE's industrial products through IoT, which was abandoned due to internal resistance from divisions reliant on service contract revenue. * **Success of Health Catalyst:** A software analytics company for hospital systems, achieving a $2.7 billion market capitalization. * **Success of Teledoc:** A virtual visit company, achieving a $22.8 billion market capitalization. * **Success of Veeva Solutions:** A cloud computing provider for the biopharma industry, founded in 2007 and achieving a $50 billion market capitalization.

QMS Software Overview of The Lean Machine - Quality, Risk, and Material Management
Total Lean Management Software
/@totalleanmanagementsoftwar8554
Aug 21, 2021
This video provides an in-depth overview of "The Lean Machine," an all-in-one Quality Management Software (QMS) solution designed to help businesses optimize resource utilization, manage risks, and ensure continuous improvement. The presenter, David, highlights how the software addresses the critical need for organizations to get and stay organized, orchestrating resources effectively to satisfy customers and drive sales, while simultaneously identifying and mitigating risks that could otherwise squander efforts. The core message emphasizes that an effective QMS is vital for business success, and The Lean Machine is engineered to enhance this system through user-friendly, comprehensive, and integrated functionalities. The presentation systematically walks through the software's key attributes, starting with its ease of implementation and intuitive user interface. It then delves into the comprehensive nature of the system, which comprises 26 modules covering quality, risk, and material management workflows. A significant focus is placed on the system's integration capabilities, demonstrating how various modules interlink to provide a holistic view of operations, from inventory management integrated with approved parts and suppliers to document control linked with ISO standards for auditor review. The speaker underscores the importance of visible accountability, achieved through user-specific dashboards that consolidate assignments and prioritize tasks with color-coded alerts. Further, the video details the robust support structure, including direct technical assistance from the development team, and advanced communication features like integrated email alerts and server-side escalation systems. The Lean Machine's ability to integrate with existing ERP systems is presented as a major advantage, allowing quality-related data to be leveraged from enterprise-wide systems. Custom development is offered as a flexible solution for unique business requirements, ensuring the software can adapt to specific organizational needs. Finally, the presentation addresses affordability through flexible pricing and a companion web app, and critically, highlights the software's strong validation support for medical device customers, boasting a 100% success rate in first-time registration audits and a commitment to evolving with regulatory standards like ISO 9001:2015. Key Takeaways: * **Strategic Resource Management:** The Lean Machine helps businesses intelligently use limited resources (time, money, knowledge, employee motivation) by getting organized, staying organized, and orchestrating tasks towards customer satisfaction and sales, while proactively managing risks. * **User-Friendly Design:** The software is designed for ease of use and rapid implementation, featuring a consistent interface across its 26 modules, with simple navigation, filtering capabilities, and tabbed record views. * **Comprehensive Modular System:** It offers a wide array of modules covering quality, risk, and material management, allowing companies to incorporate necessary quality principles, eliminate waste, and drive continuous improvement. * **Integrated Workflows:** The system provides deep integration between modules, such as linking inventory with approved parts/suppliers and inspection plans, or connecting document control to specific ISO sections for streamlined auditing and compliance. * **Visible Accountability:** User dashboards centralize assignments, linking users directly to their tasks. Priority information is color-coded based on age and system configuration, ensuring nothing falls through the cracks and critical tasks are addressed promptly. * **Robust Communication and Escalation:** An integrated email system automates communication points, such as alerting reviewers for document approvals. A server-based alerts and escalation system sends targeted emails to users based on dashboard categories, enhancing inter-user communication for quality system tasks. * **ERP System Integration:** The Lean Machine can integrate with existing ERP systems, allowing for the import of data (e.g., PO numbers) to enrich quality processes, providing robust solutions for quality-side data management that many ERPs lack. * **Custom Development Flexibility:** The vendor offers custom development projects to add unique functionalities requested by customers, often implementing minor changes free of charge and significant efforts for a modest project fee, ensuring the software evolves with user needs. * **Affordable and Scalable Pricing:** Pricing is structured to be affordable, including all modules with permission settings to control user access. Volume discounts are available, and a companion web app offers access to released documents and training sign-offs for a larger user base, reducing the need for full desktop licenses. * **Critical Validation Support for Regulated Industries:** For medical device customers, the software provides comprehensive validation documentation sets, including pre-written test protocols and online tutorials, which are robust enough to satisfy auditors and can be executed with risk-appropriate effort. * **High Audit Success Rate:** The Lean Machine boasts a 100% success rate at first-time registration audits and has successfully navigated countless customer installations, often receiving compliments from auditors who appreciate its effectiveness. * **Commitment to Evolving Standards:** The software is continuously updated to address new regulatory requirements, such as the dedicated module for risk and opportunity requirements introduced in ISO 9001:2015, ensuring ongoing compliance. * **Direct Technical Support:** Technical support is provided directly by the development team, which serves as a strong motivator for getting features right before release and ensures faster resolution of technical issues by actual experts. Tools/Resources Mentioned: * The Lean Machine QMS * ERP Systems * Active Directory Key Concepts: * **Quality Management System (QMS):** A formalized system that documents processes, procedures, and responsibilities for achieving quality policies and objectives. * **Risk Management:** The process of identifying, assessing, and controlling risks to an organization's capital and earnings. * **Material Management:** The process of planning, organizing, and controlling the flow of materials from their initial purchase through internal operations to the final product. * **Continuous Improvement:** An ongoing effort to improve products, services, or processes. * **ISO 9001:2015:** An international standard for quality management systems, which includes requirements for risk and opportunity management. * **Software Validation:** The process of ensuring that software meets its intended use and user requirements, particularly critical in regulated industries like medical devices and pharmaceuticals.

Deviations in QMS Software - The Lean Machine
Total Lean Management Software
/@totalleanmanagementsoftwar8554
Aug 19, 2021
This video provides an in-depth exploration of deviations within a Quality Management System (QMS) software, specifically showcasing "The Lean Machine." The presenter begins by defining a deviation as any departure from standard procedures or specifications that results in non-conforming material or processes, or an unusual event with the potential to impact product quality, system integrity, or personal safety. The core message emphasizes the critical importance of managing these deviations for compliance with Good Manufacturing Practices (GMP) and for fostering continuous improvement, particularly for FDA-regulated companies like those in the pharmaceutical sector. The discussion elaborates on the necessity of recording deviations in a formal deviation report. Such reports are required whenever there's a departure from methods or controls specified in manufacturing documents, material control documents, Standard Operating Procedures (SOPs), or when out-of-specification (OOS) results are confirmed. Furthermore, any event suggesting a real or potential quality-related problem, or even a noticeable trend requiring further investigation, mandates a deviation report. The video stresses that all batch production deviations—whether planned or unintended, covering manufacturing, equipment, operations, distribution, procedures, systems, and record-keeping—must be reported and investigated for corrective and preventive action (CAPA), irrespective of the final batch disposition. The video then transitions into a demonstration of "The Lean Machine" software's deviation module, positioning it as a tool that addresses the quality system requirement listed under ISO 9001: 10.2.2 for documenting non-conformances. The software allows for extensive customization of description fields and offers configurable layout options (e.g., three-field or five-field modes). The presenter highlights the straightforward workflow, where assigned deviations appear on users' dashboards, facilitating efficient management. A key design philosophy of "The Lean Machine" is its flexibility, enabling companies to use it at a minimum level of detail while still ensuring robust quality system procedures and aiding in the prevention of audit findings. Further features of the deviation module are explored, including optional links to affected procedures or parts, and two distinct options for trend analysis—one dedicated to the deviation module and another integrated with corrective actions, allowing for combined reporting. The system also supports configurable review processes, such as the ability to force at least one additional reviewer before a deviation is signed off and closed. Reporting capabilities are comprehensive, offering individual deviation reports, main menu trend reporting, and module-specific reporting screens with a variety of filters. These filters enable insightful data analysis, including the ability to report on deviations from different company locations, thus providing a holistic view of quality performance. Key Takeaways: * **Definition and Impact of Deviations:** A deviation is a critical departure from established procedures or specifications that can lead to non-conforming materials or processes, or an unexplained event with potential negative impacts on product quality, system integrity, or personnel safety, making its management crucial for GMP compliance and continuous improvement. * **Mandatory Reporting Scenarios:** Deviation reports are essential for documenting any departure from specified methods, controls, SOPs, confirmed out-of-specification (OOS) results, or any event indicating a real or potential quality problem, including the identification of adverse trends. * **Comprehensive Scope for Reporting:** All batch production deviations, encompassing all manufacturing facilities, equipment, operations, distribution procedures, systems, and record-keeping, must be reported and thoroughly investigated for Corrective and Preventive Actions (CAPA). * **Independence from Batch Disposition:** The requirement for a deviation report is absolute, regardless of the final disposition of the batch; even if a batch is rejected, the deviation must still be formally documented. * **QMS Compliance and ISO Standards:** Effective deviation management is a fundamental component of a Quality Management System (QMS), directly addressing requirements such as ISO 9001: 10.2.2, which mandates the documentation of non-conformances, their nature, subsequent actions, and results. * **Integrated Non-Conformance Management:** Robust QMS software, like "The Lean Machine," facilitates comprehensive non-conformance management by linking deviation modules with other related modules such as corrective actions, rejected materials, and customer feedback. * **Customization for Operational Fit:** QMS software should offer extensive customization options for data fields and layout configurations (e.g., three-field or five-field modes) to ensure it aligns precisely with a company's specific operational needs and desired level of detail. * **Streamlined Workflow and Accountability:** A well-designed deviation module provides a straightforward workflow, assigning deviations to responsible individuals and displaying them on dashboards for efficient tracking, completion, and approval, enhancing accountability. * **Mitigation of Audit Findings:** Implementing sound quality system procedures through comprehensive deviation management software is a proactive measure that helps companies avoid audit findings by ensuring proper documentation, investigation, and resolution of non-conformances. * **Advanced Trend Analysis Capabilities:** The ability to conduct trend analysis, either specifically for deviations or in combination with corrective actions, is vital for identifying recurring issues, understanding root causes, and driving strategic continuous improvement initiatives. * **Configurable Review and Reporting:** QMS systems should support flexible review processes, such as requiring multiple reviewers before sign-off, and offer diverse reporting functionalities, including individual reports, main menu trend reports, and module-specific screens with filters for location-based analysis, providing actionable insights. **Tools/Resources Mentioned:** * **The Lean Machine:** A Quality Management System (QMS) software demonstrated in the video for managing deviations and other non-conformances. **Key Concepts:** * **Deviation:** A departure from standard procedures or specifications, leading to non-conforming material/processes or an unusual event impacting quality, system integrity, or safety. * **Non-conformance:** A non-fulfillment of a requirement, often documented through deviation reports. * **Out-of-Specification (OOS) Results:** Test results that fall outside the established acceptance criteria for a product or process. * **Good Manufacturing Practice (GMP):** A system for ensuring that products are consistently produced and controlled according to quality standards. * **Corrective and Preventive Action (CAPA):** A system for investigating and correcting non-conforming products or processes (corrective action) and preventing their recurrence (preventive action). * **Quality Management System (QMS):** A formalized system that documents processes, procedures, and responsibilities for achieving quality policies and objectives. * **ISO 9001: 10.2.2:** A specific clause within the ISO 9001 standard that outlines requirements for documenting information about non-conformances, subsequent actions, and results of corrective actions. * **Document Management System:** A system used to store, manage, and track electronic documents and electronic images of paper-based information.

What is a Medical Device (per the EU Medical Device Regulations)?
GreenSoft Technology, Inc.
/@GreenSoftTechnology
Aug 16, 2021
This video provides an in-depth exploration of what constitutes a "medical device" under the European Medical Device Regulations (EU MDR), which became mandatory for producers on May 26, 2021. The speaker's main purpose is to clarify the precise definition of a medical device, emphasizing the manufacturer's intended purpose, and to highlight key inclusions and exclusions to help companies ensure their products are compliant with the EU MDR framework. The presentation begins by establishing a broad definition: a medical device is any instrument, apparatus, appliance, software, implant, reagent, material, or other article intended by the manufacturer to be used alone or in combination for human beings for one or more specific medical purposes. The video then systematically details these four crucial medical purposes that bring an item into scope: diagnosing, preventing, monitoring, predicting, or treating a disease or other ailment; diagnosing, monitoring, treating, or otherwise addressing an injury or a disability; investigating, replacing, or modifying the anatomy of a site or a physiological or pathological process or state in the body; and examining blood, tissue, or other specimens derived from the human body. A significant point of emphasis for the speaker is the explicit inclusion of "software" in the definition. This means that applications, such as an iPhone app that provides a risk analysis for headaches based on user input, are now considered medical devices and must comply with the stringent EU MDR processing. The video also clarifies that while products primarily using pharmacological, metabolic, or immunological means (i.e., pharmaceuticals) are not medical devices, devices that assist in their delivery or function (e.g., an insulin dispenser) are. Furthermore, specific items like birth control devices, fertility treatment devices, and those designed for cleaning, disinfection, or sterilization are explicitly listed as medical devices. The discussion also highlights that the regulation applies only to devices for human beings, excluding veterinary equipment. The video proceeds to provide practical examples to illustrate the breadth of the definition, such as pacemakers for modifying physiological processes, limb replacements for modifying anatomy, and home blood sugar monitors for in vitro examination of specimens. Finally, it lists several categories explicitly excluded from the EU MDR scope, including in vitro diagnostic medical devices (covered by IVDR), medicinal products, human blood and blood products, and food. A critical nuance is provided regarding products containing excluded substances: while the substance itself may not be a medical device, the overall product incorporating it could still be if its primary function aligns with the medical device definition. The speaker maintains a clear, illustrative approach, breaking down complex regulatory language into actionable insights for producers. Key Takeaways: * The EU MDR broadly defines a medical device as any instrument, apparatus, appliance, software, implant, reagent, material, or other article intended by the manufacturer for human beings for specific medical purposes. This "intended purpose" is the primary determinant of its regulatory status. * An item falls under the medical device definition if its intended purpose is to diagnose, prevent, monitor, predict, or treat a disease or ailment; diagnose, monitor, treat, or address an injury or disability; investigate, replace, or modify anatomy or physiological/pathological processes; or examine human body specimens. * **Crucially, software is explicitly included in the definition of a medical device.** This implies that AI solutions, chatbots, or other custom software developed by IntuitionLabs.ai for diagnostic, monitoring, or therapeutic purposes in the life sciences sector could be classified as medical devices and require EU MDR compliance. * Products whose primary intended action is achieved by pharmacological, metabolic, or immunological means (e.g., pharmaceuticals like insulin) are generally excluded from the medical device definition. However, devices that dispense or assist in the use of such products (e.g., an insulin dispenser) are considered medical devices. * Specific items like birth control devices, fertility treatment devices, and those designed for cleaning, disinfection, or sterilization of medical devices are explicitly listed as medical devices under EU MDR. * The EU MDR applies only to devices intended for *human beings*, meaning veterinary equipment is outside its scope. * Examples such as pacemakers (modifying heart rate), limb replacements (modifying anatomy), and home blood sugar monitors (in vitro examination) illustrate the diverse range of products that qualify as medical devices beyond direct disease treatment. * Several categories are explicitly excluded from EU MDR, including in vitro diagnostic medical devices (covered by IVDR), medicinal products, human blood and blood products, plasma, and cosmetic products (if covered by cosmetics regulation). Food is also excluded. * Manufacturers must carefully assess products that contain excluded substances (e.g., animal tissues). While the substance itself may not be a medical device, the overall product incorporating it could still be if its primary function meets the medical device definition. * The video emphasizes that understanding the nuances of the EU MDR definition is essential for medical device producers to ensure their products are compliant, avoiding potential regulatory pitfalls. Tools/Resources Mentioned: * GreenSoft Technology, Inc. (the channel and a provider of environmental regulation solutions) * greensofttech.com/data-services/eu-medical-device-regulation-mdr (GreenSoft's online solution for EU MDR) * greensofttech.com/videos (GreenSoft's video library) Key Concepts: * **EU MDR (European Medical Device Regulations):** The comprehensive regulatory framework governing the placing on the market and putting into service of medical devices in the European Union. * **Medical Device:** A broad term encompassing a wide array of items, including software, intended for specific medical purposes in humans, as defined by the manufacturer. * **Intended Purpose:** The manufacturer's stated objective for a product, which is the foundational element in determining if it is a medical device and its subsequent classification and regulatory pathway. * **Software as a Medical Device (SaMD):** Software that, by its intended purpose, fulfills the definition of a medical device, regardless of the platform it runs on (e.g., a smartphone app). * **IVDR (In Vitro Diagnostic Medical Devices Regulation):** A separate EU regulation specifically for devices used to examine specimens derived from the human body outside the body (in vitro). * **Pharmacological, Metabolic, or Immunological Means:** The primary modes of action for medicinal products, which differentiate them from medical devices under EU MDR. Examples/Case Studies: * **Insulin Dispenser vs. Insulin:** The dispenser is a medical device, while the insulin itself is a medicinal product. * **iPhone App for Headaches:** An app that provides a risk analysis for headaches based on user input is cited as an example of software qualifying as a medical device. * **Pacemaker:** Used as an example of a device that modifies a physiological process, thereby falling under the medical device definition. * **Limb Replacement:** An example of a device that replaces or modifies anatomy, qualifying it as a medical device. * **Home Blood Sugar Monitor:** A handheld electronic device for diabetics that examines blood specimens, illustrating a device for in vitro examination.

Value-Based Care Happened 40 Years Ago... Medicare's Prospective Payment System Explained
AHealthcareZ - Healthcare Finance Explained
@ahealthcarez
Aug 15, 2021
This video provides an in-depth exploration of the historical implementation and impact of Medicare's Prospective Payment System (PPS) in the 1980s, framing it as an early and highly effective form of value-based care. Dr. Eric Bricker, the speaker, begins by establishing the context of healthcare reimbursement prior to PPS, where Medicare paid hospitals a "cost-plus" percentage of their expenses, leading to unsustainable cost escalation. He then details how the PPS, implemented between 1983 and 1986 under a bipartisan effort, fundamentally shifted this model to a fixed payment per Diagnosis Related Group (DRG), regardless of the patient's length of stay. This change incentivized hospitals to manage resources more efficiently for specific conditions like pneumonia or hip fractures, effectively introducing a form of capitation at the individual admission level. The presentation meticulously outlines the significant outcomes of PPS implementation. Data points reveal a 17% decrease in the average length of hospital stay for Medicare beneficiaries and a 3.5% reduction in admissions and discharges. A notable example cited is the shift of 300,000 cataract surgeries annually from inpatient to universally outpatient procedures, driven purely by the change in reimbursement, not clinical necessity. Interestingly, readmission rates remained unchanged, suggesting that earlier discharges did not compromise patient safety in this regard. While 30-day post-admission mortality slightly increased by 0.6 percentage points, Dr. Bricker attributes this to a sicker patient mix being admitted, as less complex cases (like cataract surgeries) were no longer admitted, thus removing low-mortality cases from the inpatient pool. Financially, PPS dramatically slowed Medicare inflation, reducing the annual payment increase rate from 10.1% to 5.7%. Beyond the direct financial and operational impacts, the video highlights crucial changes in clinical practice and broader healthcare dynamics. Hospitals, now incentivized by fixed payments, developed better inpatient care coordination, involving multidisciplinary teams of nurses, case managers, and therapists to optimize patient flow and discharge planning for Medicare patients. This improved process, initially developed for Medicare, had a "spillover effect" on commercial insurance plans, leading to an acceleration in outpatient services for commercially insured individuals as hospitals applied their newly optimized outpatient infrastructure across all patient populations. Dr. Bricker concludes by contrasting the rapid and profound changes achieved by PPS in three years with the minimal impact of the Affordable Care Act (ACA) over eleven years. He attributes this disparity to the increased economic size of the healthcare industry (10% of the economy in the 80s vs. 18% today) and its greater lobbying power, suggesting that significant systemic, government-led change is less likely today, urging organizations to drive their own internal changes. Key Takeaways: * **Historical Precedent for Value-Based Care:** Medicare's Prospective Payment System (PPS) in the 1980s served as an early and effective model of value-based care, demonstrating that shifting payment incentives can drive significant operational changes in healthcare. * **Shift from Cost-Plus to Fixed Payments:** PPS moved from reimbursing hospitals a percentage of their costs to a fixed amount per Diagnosis Related Group (DRG), regardless of length of stay, effectively introducing capitation at the admission level. * **Significant Operational Efficiency Gains:** The implementation of PPS led to a 17% decrease in the length of hospital stays and a 3.5% reduction in admissions/discharges for Medicare beneficiaries, driven by financial incentives for efficiency. * **Impact on Clinical Practice:** Hospitals improved inpatient care coordination and discharge planning for Medicare patients, utilizing multidisciplinary teams to optimize patient flow and resource allocation. * **Shift to Outpatient Services:** Payment changes directly influenced the move of procedures like cataract surgeries from inpatient to outpatient settings, demonstrating how reimbursement models can dictate care delivery locations. * **Financial Savings for Payers:** Medicare's annual payment increase rate to hospitals was nearly halved from 10.1% to 5.7% after PPS implementation, proving its effectiveness in controlling healthcare inflation. * **Outcomes and Patient Safety:** While readmission rates remained unchanged, a slight increase in 30-day post-admission mortality was observed, attributed to a sicker inpatient population after less complex cases moved to outpatient settings. * **Spillover Effect on Commercial Insurance:** The operational efficiencies and outpatient infrastructure developed for Medicare patients under PPS were subsequently applied to commercially insured patients, accelerating the increase in outpatient services across the board. * **Difficulty of Systemic Change Today:** The speaker posits that the healthcare industry's increased economic size (18% of the economy today vs. 10% in the 80s) and lobbying power make large-scale, government-mandated systemic changes much harder to implement effectively compared to the 1980s. * **Call for Internal Organizational Change:** Given the perceived inertia at the governmental level, organizations within the healthcare ecosystem are encouraged to proactively drive their own changes and efficiencies rather than waiting for top-down systemic reforms. Tools/Resources Mentioned: * **AHealthcareZ.com:** The channel's website for healthcare finance educational videos and newsletters. * **16 Lessons in the Business of Healing:** Dr. Bricker's book. * **Steve Brill's Book 'America's Bitter Pill':** Referenced for insights into the healthcare industry's influence on government. * **NCBI and Princeton University Articles:** Academic sources cited for data and analysis on PPS. Key Concepts: * **Prospective Payment System (PPS):** A method of reimbursement in which Medicare payment for hospital inpatient services is a fixed amount per diagnosis, regardless of the actual cost or length of stay. * **Diagnosis Related Groups (DRGs):** A classification system that categorizes patients into groups based on diagnosis, procedures, age, and other factors, used to determine the fixed payment amount under PPS. * **Value-Based Care:** A healthcare delivery model where providers are paid based on patient health outcomes, rather than the volume of services provided. The video frames PPS as an early, albeit unnamed, form of this. * **Cost-Plus Reimbursement:** A payment model where providers are reimbursed for their costs plus an additional percentage, which can incentivize higher spending. * **Capitation:** A payment arrangement where a healthcare provider is paid a fixed amount per patient per period of time, regardless of how many services the patient uses. PPS applied this concept at the individual admission level.

Enterprise Quality Management System | Qualityze EQMS Software
Qualityze
/@Qualityze
Aug 10, 2021
This video introduces Qualityze Enterprise Quality Management System (EQMS), positioning it as an essential tool for businesses operating in highly regulated and rapidly evolving environments. The core message emphasizes that innovation is the leading factor for survival and success, requiring enterprises to adopt smarter tools to manage business processes, enhance product quality, and ensure robust collaboration. Qualityze aims to transform traditional Quality Management Systems (QMS) into a "Quality Decision Making Engine," shifting the focus from mere documentation to actionable, data-driven quality improvement. The presentation highlights the critical role of quality in differentiating products and ensuring market success. Qualityze asserts that its solutions are designed to ensure that every product delivered makes a difference, every implemented change triggers quality improvements, and every best-practice workflow meets stringent compliance standards. This focus on regulatory adherence and continuous improvement is central to the platform’s value proposition, particularly for industries facing strict regulatory oversight. The video underscores the need for digital maturity in quality management, moving away from fragmented or manual processes toward integrated, cloud-based solutions. A significant aspect of the Qualityze offering is its technological foundation and future-proofing capabilities. The EQMS is explicitly built on the globally trusted Salesforce.com cloud platform, providing inherent scalability, security, and integration potential. Furthermore, Qualityze positions itself as an innovation leader by supporting major business evolutions and technological advancements, including Artificial Intelligence (AI), Machine Learning (ML), the Internet of Things (IoT), Industry 4.0, Quality 4.0, and cloud computing. This integration capability allows the EQMS to serve as a central hub for quality data, ready to leverage advanced analytics and AI for predictive quality and automated compliance tracking. The comprehensive suite of modules—covering areas like CAPA, Nonconformance, Document Control, and Audit Management—demonstrates its capability to manage the entire quality lifecycle within a single, integrated platform. Key Takeaways: * **Innovation in Quality Management is Crucial:** Businesses must embrace innovation not just in product development but also in process management to survive competitive pressures and adapt to evolving customer and regulatory requirements. Qualityze positions its EQMS as the necessary innovation for quality processes. * **Focus on Compliance and Quality Improvement:** The EQMS is designed to ensure that quality remains the primary focus, guaranteeing that every change implemented triggers measurable quality improvements and that all best-practice workflows are compliant with industry regulations. * **Salesforce Platform Foundation:** Qualityze is built on the Salesforce.com cloud platform, offering inherent benefits such as reliability, scalability, security, and ease of integration with other enterprise systems commonly used in the life sciences sector (e.g., Veeva CRM). * **Transformation to a Decision-Making Engine:** The goal of the Qualityze EQMS is to move beyond being a passive record-keeping system (QMS) to becoming an active "Quality Decision Making Engine," implying the use of data and intelligence to guide strategic quality choices. * **Support for Advanced Technologies:** The platform is engineered to support and integrate with modern technological evolutions, specifically mentioning Artificial Intelligence, Machine Learning, IoT, Industry 4.0, and Quality 4.0. This makes the system future-proof and ready for AI-driven automation and predictive quality initiatives. * **Comprehensive Quality Lifecycle Management:** The Qualityze suite covers a wide range of critical GxP and regulatory processes, including Nonconformance Management, Corrective and Preventive Action (CAPA) Management, Document Control, Change Management, Supplier Quality, and Audit Management. * **Digital Maturity through Cloud Solutions:** Utilizing a cloud-based solution helps enterprises achieve digital maturity in their quality processes, replacing disparate or paper-based systems with a centralized, collaborative digital environment. * **Targeted at Regulated Industries:** The explicit mention of catering to industries with "strict regulatory requirements" confirms the platform’s suitability and focus on sectors like pharmaceuticals, biotech, and medical devices, where GxP compliance is mandatory. Tools/Resources Mentioned: * **Qualityze EQMS Software:** The core Enterprise Quality Management System product. * **Salesforce.com:** The trusted global cloud platform upon which the Qualityze software is built. * **Specific EQMS Modules:** Nonconformance Management, CAPA Management, Document Control Management, Change Management, Supplier Quality Management, Audit Management, Employee Training Management, Customer Complaints Management, Maintenance Management, Calibration Management, Inspection Management, Permit Management, and Material Compliance Management. Key Concepts: * **Enterprise Quality Management System (EQMS):** A centralized software system used to manage and automate quality-related processes across an organization, ensuring compliance and driving continuous improvement. * **Quality 4.0:** The application of advanced technologies (AI, ML, IoT, Cloud) to quality management processes, moving from reactive quality control to predictive and proactive quality assurance. * **Digital Maturity:** The level of digital integration and optimization within an organization's processes, enabling efficient, data-driven operations. * **Compliance:** Adherence to external regulatory standards (e.g., FDA, EMA, GxP), which is a core function of the EQMS, particularly through modules like Document Control and Audit Management.

Re-Imagine your Veeva Validation Strategy with Opkey’s Automated Continuous Validation platform
Opkey
/@Opkey
Aug 9, 2021
This video provides an overview of how automated continuous validation platforms can transform the testing and compliance strategy for pharmaceutical companies utilizing Veeva systems. The core message centers on mitigating the regulatory burden and risks associated with Veeva configuration changes and updates by shifting from manual, periodic validation to an automated, continuous process. The speaker emphasizes that the current environment, accelerated by global events, necessitates a fundamental rethinking of traditional testing strategies to ensure ongoing compliance with applicable regulations, a critical requirement for GxP systems like Veeva CRM. The solution presented focuses on proactively addressing common Veeva testing challenges, particularly the configuration validation burden that often slows down deployment and integration. By leveraging an automated platform, organizations can significantly reduce the time and effort required for validation. A key component of this strategy involves using pre-built validation packs, which allow organizations to achieve up to 70% faster validation cycles. This automation extends to seamlessly managing Process Qualification Status (PQS) and regression test suites, ensuring that every system change is thoroughly tested without extensive manual intervention. A central technical feature highlighted is the use of smart Business Configuration Document (BCD) adapters, which are instrumental in automating configuration validation. This technology enables 100% data validation coverage with every system update. Furthermore, the platform incorporates real-time, risk-based impact analysis. This capability allows testing teams to identify exactly which parts of the system are affected by a change, enabling them to conduct the minimum necessary number of tests while achieving maximum coverage. This targeted approach is claimed to save an average of six weeks in testing time. Finally, the platform ensures comprehensive visibility through detailed standard and custom validation reports, helping organizations maintain an "always validated state" and strengthen adherence to regulatory demands. Key Takeaways: • **Automated Continuous Validation is Essential for Compliance:** The traditional, manual testing strategy is insufficient for maintaining compliance in dynamic environments like Veeva, necessitating a shift to automated continuous validation to proactively address regulatory demands and mitigate operational risks. • **Significant Reduction in Validation Burden:** Implementing automated configuration validation solutions drastically reduces the manual effort associated with validating Veeva configurations, freeing up specialized resources for more strategic tasks. • **Achieve 70% Faster Validation Cycles:** By utilizing pre-built validation packs, organizations can accelerate their overall validation process, enabling quicker deployment of new Veeva features and configurations while maintaining regulatory rigor. • **Seamless Automation of PQS and Regression Suites:** The platform supports the seamless automation of both Process Qualification Status (PQS) and standard regression testing, ensuring that all critical business processes remain functional and compliant after system updates. • **100% Data Validation Coverage:** Automated tools ensure complete data validation coverage with every Veeva update, eliminating blind spots and ensuring data integrity, which is crucial for GxP compliance. • **Real-Time Risk-Based Impact Analysis:** This capability allows testing teams to efficiently determine the scope of testing required following a change, focusing resources only on impacted areas and avoiding unnecessary full-suite testing. • **Average Six Weeks Time Savings:** The combination of automated testing and risk-based impact analysis ensures that organizations conduct the minimum number of tests for maximum coverage, resulting in substantial time savings, estimated at an average of six weeks per validation cycle. • **Smart BCD Adapters Drive Configuration Automation:** Specific adapters designed for Business Configuration Documents (BCDs) are key to automating the validation of Veeva configuration settings, ensuring accuracy and consistency across environments. • **Maintain an "Always Validated State":** The goal of continuous validation is to move beyond periodic validation checkpoints to a state where the system is perpetually compliant and ready for audit, significantly strengthening regulatory posture. • **Enhanced Visibility through Reporting:** The platform provides 100% visibility into the validation status through detailed standard and custom reports, which are essential for audit trails and demonstrating compliance to regulatory bodies. Tools/Resources Mentioned: * **Opkey:** The automated continuous validation platform. * **Veeva:** The enterprise platform (implied Veeva CRM) requiring validation. * **Pre-Built Validation Pack:** A resource provided by the platform to accelerate initial validation setup. * **Smart BCD Adapters:** Specific tools used for automating the validation of Veeva Business Configuration Documents. Key Concepts: * **Continuous Validation:** A methodology where validation activities are integrated throughout the software development and deployment lifecycle, ensuring that the system remains in a validated state continuously, rather than only after major updates. * **Risk-Based Impact Analysis:** A technique used to analyze the potential impact of a system change and determine the minimum set of tests required to confirm system integrity and compliance, maximizing coverage while minimizing testing time. * **PQS (Process Qualification Status):** Refers to the qualification and validation of the specific business processes executed within the regulated system, a critical component of GxP compliance. * **Veeva Configuration Validation:** The process of ensuring that the specific settings, customizations, and configurations made within the Veeva platform meet regulatory requirements and function as intended.

Veeva Vault AUI
LPW Talk Time and Learning
/@lpwtalktimeandlearning4213
Aug 6, 2021
This video provides an in-depth overview of the upcoming Veeva Vault Action User Interface (AUI) update, detailing its new look and feel, release schedule, and the critical steps organizations need to take for a smooth transition. The presenter emphasizes that Veeva has given Vault a "new facelift," resulting in a more modern, streamlined interface designed to enhance user experience and efficiency by keeping frequently accessed workflows readily available. This update is presented as more than just a cosmetic change, aiming to make the system easier and more intuitive for users. The core of the AUI update involves significant changes to navigation and interaction elements. Specifically, the traditional action menu will be replaced by a more contemporary ellipses icon, streamlining the visual interface. Furthermore, numerous common Vault actions, document actions, and object actions will be represented by new, modern icons, all contributing to a more intuitive and efficient user experience. The video highlights that these changes are intended to simplify user interaction and improve overall productivity within the Veeva Vault environment. Regarding the rollout, Veeva has scheduled a general release for the Action UI on August 6th, at which point the feature will be visible to all users if activated. However, customers have the flexibility to delay their adoption of the new interface until December 3rd. Regardless of the chosen timeline, the video stresses the imperative for organizations to prepare adequately. Key preparation steps include carefully selecting an adoption timing that aligns with internal operational needs, developing a robust communication plan to inform employees about the upcoming changes, and critically, refreshing all existing training materials, including videos and e-learning modules, to reflect the new interface. The speaker's company, LPW Training, positions itself as a partner in this transition, viewing the UI update as an opportune moment to not only update but also streamline and modernize existing training programs to match the new visual and functional experience of Veeva Vault. Key Takeaways: * **Significant UI Overhaul:** Veeva Vault is undergoing a major user interface update called Action UI, which introduces a modern, streamlined look and feel designed to improve user experience and operational efficiency. * **Enhanced User Experience Focus:** The primary goal of the AUI is to make the system easier and more efficient to use by keeping frequently accessed workflows a click away and updating visual cues. * **Key Interface Changes:** Specific UI modifications include the replacement of the traditional action menu with an ellipses icon and the introduction of several new, modern icons for common Vault, document, and object actions. * **Flexible Adoption Timeline:** Organizations have two main options for adopting the AUI: an early general release on August 6th, or a delayed adoption option until December 3rd, allowing companies to choose the timing that best suits their internal readiness. * **Mandatory Preparation:** Regardless of whether an organization chooses early or delayed adoption, comprehensive preparation is essential to ensure a smooth transition and minimize disruption to user workflows. * **Strategic Timing Selection:** A critical first step in preparation is to carefully select the adoption timing that aligns with the company's operational schedule, resource availability, and change management capacity. * **Robust Communication Plan:** Developing and executing a clear communication plan is vital to inform employees about the upcoming changes, manage expectations, and facilitate user acceptance of the new interface. * **Critical Training Material Refresh:** All existing training materials, including videos and e-learning modules, must be updated to reflect the new screens, icon placements, and overall look and feel of Veeva Vault AUI to ensure users receive accurate and effective guidance. * **Opportunity for Training Modernization:** The AUI update presents a unique opportunity for companies to not just update, but also to streamline and modernize their entire Veeva Vault training program, aligning it with current best practices in learning and development. * **External Support Available:** Specialized firms like LPW Training offer assistance with various aspects of the AUI transition, including help with communication strategies, refreshing current training materials, and reimagining comprehensive Vault training programs. Tools/Resources Mentioned: * Veeva Vault * Action UI (AUI) Key Concepts: * **Action UI (AUI):** The new user interface for Veeva Vault, characterized by a modern, streamlined design and updated icons. * **Early Adopter:** Refers to organizations that choose to implement the Veeva Vault AUI immediately upon its general release on August 6th. * **Delayed Adoption:** Refers to organizations that opt to postpone the implementation of the Veeva Vault AUI until December 3rd, allowing for more preparation time. * **Communication Plan:** A structured strategy for informing employees about upcoming system changes, managing expectations, and providing necessary support. * **Training Materials Refresh:** The process of updating existing instructional content (e.g., videos, e-learns) to accurately reflect changes in a software's user interface and functionality.

Doctor Specialties That Have Power at Hospital Systems
AHealthcareZ - Healthcare Finance Explained
@ahealthcarez
Aug 1, 2021
This video provides an in-depth exploration of the financial power dynamics within major hospital systems, identifying specific physician specialties that drive the majority of hospital revenue and influence. Dr. Eric Bricker, Chief Medical Officer of a Value-Based Care Company, frames the discussion by recounting an offer to become a Chief Medical Officer of Value-Based Care at a top hospital system, immediately questioning the actual influence such a role could wield given the established financial incentives. He posits that the administration of hospital systems maintains a highly symbiotic relationship with particular physician groups, not based on medical necessity alone, but primarily on their ability to generate high-margin services. The core of the video details how Orthopedics, Neurosurgery (specifically spine), Cardiology, and Oncology are the "royalty" within hospital systems. These specialties are highlighted for performing complex, high-cost procedures on commercially insured patients, such as knee and hip surgeries, spine surgeries, cardiac cath lab procedures, nuclear stress tests, and inpatient chemotherapy. These services are presented as the primary drivers of hospital growth and profitability. Hospitals, therefore, go to great lengths to attract and retain these specialists, offering incentives like co-owned ambulatory surgery centers and facility fee sharing to ensure their continued presence and productivity. In contrast, other specialties like ENT, Urology, Vascular Surgery, Ophthalmology, and OB/GYN are categorized as mid-tier, often dealing with lower-paying Medicare or Medicaid populations, or performing procedures that yield less significant margins. Specialties such as Psychiatry, Primary Care Physicians, ER physicians, and Radiologists are deemed to hold minimal power, often seen as cost centers or easily replaceable. A significant portion of the video is dedicated to explaining why value-based care (VBC) models inherently conflict with this established fee-for-service financial structure. Dr. Bricker argues that VBC, by design, "betrays" the high-margin specialties by reducing patient volume and procedure counts. This reduction occurs through mechanisms like increased referrals to physical therapy by primary care physicians, decreased complex imaging, fewer emergency room visits, and more effective cancer screening leading to earlier-stage diagnoses that require less intensive (and less profitable) treatments like chemotherapy. The speaker concludes that the vast majority of hospital systems are unwilling to adopt VBC widely because it directly undermines the financial stability provided by these high-margin specialties. A rare successful example of VBC implementation involved hospitals paying these specialists *more* to perform *fewer* procedures, essentially buying them out of the fee-for-service model to align incentives. Key Takeaways: * **Financial Drivers of Hospital Power:** The true power dynamics within hospital systems are dictated by physician specialties that generate high-margin revenue, primarily Orthopedics, Neurosurgery (spine), Cardiology, and Oncology. * **High-Margin Procedures:** These dominant specialties drive profitability through expensive procedures like knee/hip surgeries, spine surgeries, cardiac cath lab interventions, nuclear stress tests, and inpatient chemotherapy, especially when performed on commercially insured patients. * **Hospital Incentives for Specialists:** Hospitals actively court and retain high-margin specialists by offering significant incentives, including co-ownership opportunities in ambulatory surgery centers and sharing facility fees to ensure their continued loyalty and productivity. * **Hierarchy of Influence:** A clear hierarchy exists where high-margin specialists are treated as "royalty," while specialties like Psychiatry, Primary Care, and Emergency Room physicians hold minimal sway, often viewed as cost centers or easily replaceable. * **Value-Based Care Conflict:** Value-Based Care (VBC) models fundamentally conflict with the fee-for-service incentives of high-margin specialties by aiming to reduce patient volume and procedure counts through preventative care and conservative treatments. * **Impact of VBC on Procedure Volume:** VBC initiatives lead to decreased complex imaging, increased utilization of physical therapy, and fewer ER visits, directly impacting the revenue streams of orthopedic, neurosurgery, and cardiology departments. * **Oncology Revenue Implications:** In oncology, VBC's emphasis on early cancer screening results in detecting earlier-stage cancers (e.g., DCIS, pre-cancerous polyps) that often do not require chemotherapy, thereby reducing high-margin treatment volumes for oncologists. * **Hospital Resistance to VBC:** Most hospital systems are reluctant to fully embrace VBC because it directly threatens the financial viability and profitability derived from their high-margin, fee-for-service specialties. * **Cost of VBC Transition:** A rare successful strategy for transitioning high-margin specialists to VBC involves paying them *more* to perform *fewer* procedures, effectively compensating them for lost fee-for-service revenue to align with value-based goals. * **Intermountain Health Case Study:** The experience at Intermountain Health with its patient-centered medical home demonstrated a tangible reduction in imaging, orthopedic/neurosurgery procedures, and ER visits, validating the volume-reduction effect of VBC. * **Strategic Implications for Life Sciences:** Pharmaceutical and medical device companies must understand these hospital financial dynamics and physician power structures to effectively tailor their commercial strategies, market access approaches, and product messaging when engaging with high-value specialties and hospital systems. * **Commercial Operations Insight:** For commercial operations, recognizing which specialties are critical revenue drivers for hospitals can inform sales targeting, resource allocation, and partnership strategies, especially when considering the potential shifts introduced by value-based care. * **Regulatory and Operational Challenges:** The inherent conflict between fee-for-service profitability and value-based care goals presents significant operational and potentially regulatory challenges for hospitals, impacting how new technologies and treatments are adopted. **Key Concepts:** * **High-Margin Specialties:** Physician groups (Orthopedics, Neurosurgery Spine, Cardiology, Oncology) that generate substantial profit for hospitals due to expensive procedures performed on commercially insured patients. * **Fee-for-Service (FFS):** A traditional payment model in healthcare where providers are reimbursed for each service they perform, incentivizing higher volumes of care. * **Value-Based Care (VBC):** A healthcare delivery model where providers are paid based on patient health outcomes, quality of care, and cost-efficiency, rather than the volume of services. * **Commercially Insured Patients:** Patients covered by private health insurance plans, which typically offer higher reimbursement rates to hospitals and providers compared to government programs like Medicare or Medicaid. * **Ambulatory Surgery Centers (ASCs):** Outpatient facilities where surgical procedures are performed, often co-owned by hospitals and physicians to share facility fees and increase profitability. **Examples/Case Studies:** * **Intermountain Health:** The video references Intermountain Health's "third-generation patient-centered medical home" as a real-world example where the implementation of value-based care principles led to a measurable decrease in imaging, orthopedic/neurosurgery procedures, and ER visits, illustrating the direct impact of VBC on procedure volume.