Videos
Browse videos by topic
All Videos
Showing 97-120 of 1435 videos

Veeva's Numbers: My Take on Growth & That Litigation Charge
Corporate Decoder
/@CorporateDecoder
Sep 3, 2025
This video provides an in-depth financial and operational analysis of Veeva Systems Inc., based on its 10-Q SEC filing for the three months ending July 31, 2025. The core purpose of the analysis is to peel back the layers of the corporate filing to assess Veeva's financial health, growth trajectory, and critical business risks. The speaker, an expert content analyst, adopts a data-driven approach, systematically reviewing the balance sheet, income statement, and risk factors to provide a comprehensive view of the company's standing within the life sciences technology sector. The analysis begins by establishing Veeva’s exceptional financial stability. As of the reporting date, Veeva was sitting on nearly $2 billion in cash and cash equivalents, coupled with over $4.4 billion in short-term investments, resulting in total current assets approaching $7 billion. This massive liquidity provides a significant buffer against economic volatility. Moving to the income statement, the company reported total revenues exceeding $789 million for the quarter, marking a strong 17% year-over-year growth. Crucially, the majority of this revenue—nearly $659 million—is derived from subscription services, underscoring the predictability and recurring nature of Veeva's income stream. The company maintains a highly profitable operational structure, boasting a total gross margin percentage of 75%. A key focus of the analysis is the impact of a significant, non-recurring expense. While Veeva demonstrated efficiency by slightly reducing R&D and Sales & Marketing expenses as a percentage of revenue, General and Administrative (G&A) expenses jumped from 9% to 12% of revenue. This increase was directly attributed to a substantial $30.6 million litigation settlement charge. The speaker characterizes this charge as a "hiccup" or a one-off event, noting that despite this impact, the net income still grew by $29 million year-over-year to a cool $200 million, confirming the underlying strength of the core business driven by both commercial and R&D solutions. The final segment of the analysis delves into the critical risks facing Veeva, which are highly relevant to the broader life sciences ecosystem. These risks include intense competition from major players like Salesforce and IQVIA, the ongoing challenge of migrating customers to new platforms such as Vault CRM (a process that carries the risk of customer disruption or loss), and the inherent vulnerability associated with handling sensitive customer data (security breaches). Most importantly for the life sciences industry, the analysis highlights Veeva's concentrated reliance on this single sector. This means that adverse regulatory changes (e.g., FDA/EMA), shifts in drug pricing, or government funding fluctuations can directly and severely impact Veeva's revenue and growth prospects. Key Takeaways: • Veeva Systems exhibits exceptional financial resilience, holding nearly $7 billion in liquid assets (cash and short-term investments), which provides a strong foundation for continued platform development and market dominance in the life sciences sector. • The company’s core business is robust, demonstrated by a 17% year-over-year revenue growth and a high 75% gross margin, confirming the sustained investment appetite within pharmaceutical commercial and R&D operations. • The $30.6 million litigation settlement charge is identified as a significant, yet likely non-recurring, expense that temporarily inflated G&A costs; this event should not be viewed as indicative of a long-term decline in operational profitability. • Growth in subscription services revenue is being driven by both Commercial and R&D solutions, signaling that IntuitionLabs should continue to focus equally on optimizing both sides of the pharmaceutical business (e.g., Medical Info Chatbots and clinical data management). • The acknowledged challenge of migrating customers to new platforms, specifically Vault CRM, presents a direct, high-value opportunity for specialized consulting firms to offer expertise in complex system integration, data migration, and change management to ensure seamless adoption. • Competition from tech giants like Salesforce and industry peers like IQVIA remains a constant threat; this necessitates that IntuitionLabs differentiate its Veeva consulting services by integrating advanced AI and LLM capabilities to provide unique value beyond standard implementation. • Veeva’s reliance on a few major customers underscores the high-stakes nature of enterprise engagements in the life sciences sector; successful, long-term partnerships with these key clients are crucial for the stability of the entire Veeva ecosystem. • The concentration of revenue within the life sciences industry means that regulatory and political risks (FDA compliance, drug pricing) are paramount; all custom AI and software solutions developed by IntuitionLabs must prioritize and seamlessly integrate regulatory compliance features. • Veeva’s continued heavy investment in R&D ($92 million in the quarter) confirms a commitment to innovation, requiring consulting partners to maintain deep expertise in emerging Veeva features and API integrations, particularly those related to AI enablement. • The risk of security breaches and unauthorized data access is a top concern for Veeva, reinforcing the need for IntuitionLabs to design all custom software and data engineering solutions with stringent security protocols compliant with GxP and 21 CFR Part 11 standards. Key Concepts: * **10-Q Filing:** A comprehensive quarterly report submitted by public companies to the SEC, providing detailed financial performance data. * **Subscription Services Revenue:** Recurring income derived from software licenses and support, which forms the backbone of Veeva's predictable revenue model. * **Gross Margin:** The percentage of revenue remaining after deducting the cost of revenues (75% for Veeva), indicating high efficiency in delivering core services. * **Vault CRM:** Veeva's next-generation CRM platform, the migration to which is cited as a significant operational challenge and risk factor. * **Litigation Settlement Charge:** A one-time expense ($30.6 million) related to resolving legal disputes, impacting the reported net income for the quarter.

Medical Fraud Waste and Abuse Explained
AHealthcareZ - Healthcare Finance Explained
@ahealthcarez
Sep 1, 2025
This video provides an in-depth explanation of medical fraud, waste, and abuse (FWA), a significant issue estimated by the FBI to account for 3-10% of all healthcare spending. Dr. Eric Bricker, the speaker, breaks down FWA into four distinct categories as defined by the Centers for Medicare and Medicaid Services (CMS): mistakes, waste, abuse, and deliberate fraud, focusing on their impact on employer-sponsored health plans. He emphasizes that while insurance carriers are theoretically responsible for catching FWA, their automated claims processing and historical refusal to allow external audits often lead to substantial financial losses for self-funded employers. The presentation delves into each FWA category with vivid, real-world examples. "Mistakes" are illustrated by a $250,000 infusion medication claim that was double-billed to both a medical plan and a pharmacy benefits manager (PBM) by the same carrier, resulting in a half-million-dollar payout for a quarter-million-dollar service. "Waste" is exemplified by a healthy, normal-risk pregnant woman receiving monthly ultrasounds without clinical indication. "Abuse" is explained through "upcoding," where conditions like pneumonia or urinary tract infections were re-coded as sepsis to secure higher Medicare and commercial plan reimbursements, despite no change in patient management or hospital costs. The most egregious category, "deliberate fraud," is highlighted with two impactful case studies reported by ProPublica. One involves a personal trainer for Southwest Airlines employees who billed over $4 million in physical therapy claims over several years, despite not being a licensed physical therapist. Another details out-of-network physical therapists in New Jersey charging $667 per "medical massage" to a state employee health plan with rich out-of-network benefits, a clear scam. Dr. Bricker points out that carriers often auto-adjudicate claims under $10,000-$15,000 without human review, allowing such fraud to persist, particularly in self-funded plans where the carrier bears no risk. He also addresses the common counter-argument that prior authorizations balance out FWA, illustrating with a diagram how both problems can co-exist and impact different providers, meaning neither should be ignored. The video concludes by offering a concrete solution: independent auditing. Dr. Bricker advocates for employers and benefits consultants to hire separate data analytics firms specifically to review claims for FWA, thereby auditing the carrier's performance. He cites a benefits consultant who reduced health plan costs by 6% for large Fortune 500 companies by strictly addressing FWA. Another example includes an insurance captive that has kept healthcare costs flat for over a decade by employing two outside data analytics vendors to scrutinize carrier data. This "trust but verify" approach, enabled by demanding data access from carriers or switching to those who comply, is presented as a proven method to reclaim significant healthcare spending. Key Takeaways: * **Significant Financial Impact of FWA:** Medical Fraud, Waste, and Abuse (FWA) accounts for an estimated 3-10% of all healthcare spending, representing a substantial financial drain on health plans, including employer-sponsored ones. * **Four Categories of FWA:** CMS categorizes FWA into Mistakes (e.g., double billing), Waste (e.g., unnecessary procedures), Abuse (e.g., upcoding for higher reimbursement), and Deliberate Fraud (e.g., billing for services not rendered or by unqualified personnel). * **Carrier Limitations in FWA Detection:** Traditional insurance carriers often fail to detect FWA due to automated claims adjudication processes for claims under $10,000-$15,000 and a historical reluctance to allow external audits of their FWA departments. * **"Trust But Verify" is Essential:** For self-funded plans, carriers bear no risk for FWA, making it crucial for employers to implement a "trust but verify" strategy rather than solely relying on carrier assurances. * **FWA and Prior Authorization Are Separate Issues:** The existence of egregious prior authorization denials does not negate the need to address FWA; both are distinct problems that require separate solutions and can impact different providers. * **Independent Data Analytics as a Solution:** The most effective strategy to combat FWA is to hire independent, third-party data analytics firms to review claims data and audit the carrier's FWA detection efforts. * **Actionable Steps for Employers:** Employers should demand access to their claims data for independent review. If a carrier refuses, they should consider issuing an RFP (Request for Proposal) to find a new carrier that allows such audits. * **Tangible Cost Savings:** Addressing FWA through independent auditing can lead to significant cost reductions, with one benefits consultant reporting a 6% decrease in health plan costs for large employers. * **Empowering Claims Management:** With independent data analysis, employers can instruct carriers to stop payments on fraudulent claims or withhold future payments to providers who have previously received fraudulent payouts. * **Proven Success Models:** Examples like an insurance captive maintaining flat healthcare costs for over a decade by utilizing multiple outside FWA auditing vendors demonstrate the efficacy of this approach. * **Data Access is Paramount:** The ability to access and analyze comprehensive claims data is fundamental for identifying patterns of FWA that automated carrier systems often miss. **Key Concepts:** * **FWA (Fraud, Waste, and Abuse):** A broad term encompassing intentional deception (fraud), inefficient or unnecessary use of resources (waste), and practices that directly or indirectly result in unnecessary costs (abuse). * **Upcoding:** Billing for a more expensive service or diagnosis than what was actually provided or justified, often to increase reimbursement. * **Auto-adjudication:** The automated processing and payment of claims by an insurance carrier's computer system without human review, typically for claims below a certain monetary threshold. * **Self-funded Plan:** An employer-sponsored health plan where the employer directly pays for employees' healthcare costs rather than paying premiums to an insurance carrier, making them directly responsible for FWA losses. * **Prior Authorization:** A requirement from an insurance plan that a healthcare provider obtain approval before providing a service or prescribing a medication, often used to control costs and ensure medical necessity. * **RFP (Request for Proposal):** A document issued by an organization to solicit bids from potential suppliers for a specific project or service, used here to find carriers willing to allow independent FWA audits. **Examples/Case Studies:** * **Double-billed Infusion Medication:** A $250,000 infusion drug was billed twice (medical and pharmacy claims) by the same carrier/PBM, resulting in a $500,000 payout for a $250,000 service. * **Unnecessary Monthly Ultrasounds:** A healthy, normal-risk pregnant woman received monthly ultrasounds without clinical indication, representing medical waste. * **Sepsis Upcoding:** A significant increase in sepsis diagnoses (from 248,000 to 541,000 cases) was observed after Medicare increased reimbursement, with conditions like pneumonia being re-coded as sepsis to gain higher payments without changes in patient care. * **Southwest Airlines Personal Trainer Fraud:** A personal trainer billed Southwest Airlines' employee health plan over $4 million for physical therapy claims, despite not being a physical therapist. * **New Jersey Out-of-Network Massage Fraud:** Out-of-network physical therapists in New Jersey billed $667 per "medical massage" to a state employee health plan with generous out-of-network benefits, amounting to significant fraud. * **Benefits Consultant Cost Reduction:** A benefits consultant achieved a 6% reduction in health plan costs for Fortune 500 employers by rigorously addressing FWA through independent auditing. * **Insurance Captive Flat Costs:** An insurance captive maintained flat healthcare costs for over 10 years by employing two outside data analytics vendors to audit carrier claims for FWA.

Season 2, Episode 5: The Heart of the TMF with Ann Ackley-Fifer
Heart of the Trial
/@HeartoftheTrial
Sep 1, 2025
This episode features an in-depth discussion with Ann Ackley-Fifer, a TMF expert and consultant, exploring the evolution of the Trial Master File (TMF) from paper-based systems to modern digital strategies. The conversation emphasizes the TMF’s role as the "heart of the trial," detailing Ann’s nearly 20-year journey from manually digitizing paper documents to becoming a Veeva-certified expert focused on system configuration and end-user empowerment. A central theme is the necessity of foundational TMF knowledge, arguing that while technology (including AI and machine learning) can handle the 'what' (data collection and synthesis), human expertise is required for the 'why' (context, patient focus, and regulatory interpretation). The discussion heavily focuses on the impact of regulatory changes and the shift toward digital compliance. A significant portion addresses the implications of ICH GCP E6(R3), specifically highlighting the increased emphasis on sponsor oversight and the need for greater transparency from Contract Research Organizations (CROs). Ann argues that E6(R3) gives sponsors the necessary leverage to demand visibility into in-progress documents, missing items, and quality control metrics, preventing CROs from guarding messy data until the last minute. The speakers stress that inspection readiness must be a continuous mindset, not a periodic activity, noting that regulators are increasingly demanding direct access to electronic TMF (eTMF) systems and are adept at identifying last-minute cleanup efforts via audit trails. Furthermore, the conversation delves into the future of TMF management, emphasizing the move toward standardized, digitally native documentation. Ann highlights the work being done by CDISC with the M11 standardized digital protocol and the TMF Reference Model version 4, which aims for data in a system to print out a document narrative, rather than documents being mined for data. This shift is seen as crucial for enabling cooperation among smaller biotech companies by ensuring their systems speak the same language and collect standardized data points. However, a major concern raised is the potential loss of fundamental TMF knowledge as entry-level roles become obsolete due to decentralization and automation, stressing the need for TMF champions within functional areas to maintain oversight and context. Ann’s consulting philosophy centers on the end-user experience, recognizing that even the most sophisticated eTMF system fails without practical training and user understanding. She notes that many consulting engagements, initially focused on technology configuration, quickly pivot to addressing end-user education and process adoption. The goal is to demonstrate the tangible benefits of proper documentation—such as using system filtration to generate a list of expiring documents for a site monitor—to create an "aha moment" that encourages consistent compliance. The ultimate message is that TMF integrity is paramount because it represents the complete story of the trial, and any gaps or inconsistencies raise red flags for inspectors regarding the integrity of the entire study. ### Detailed Key Takeaways * **Inspection Readiness is a Mindset, Not an Activity:** TMF compliance should be a continuous process, not a quarterly or biannual cleanup effort. Regulators are aware of last-minute pushes; audit trails expose when a high volume of documents were QC’d or logged just weeks before an inspection, which serves as a major red flag. * **ICH GCP E6(R3) Demands Transparency:** The updated guidelines place a strong focus on sponsor oversight, empowering sponsors to demand visibility into their CROs' TMFs. CROs must move beyond only showing approved documents and provide insight into in-progress items, missing documents, and quality control trends to remain competitive. * **The Importance of Foundational TMF Knowledge:** As technology automates entry-level tasks, there is a risk of losing the "detective" skills gained by early TMF professionals who learned the 'why' and context of documents by reverse-engineering them. Technology is a research assistant, not the professor; human expertise is needed to connect the dots across functional areas. * **Metrics Must Be Straightforward:** If a CRO's TMF metrics require extensive caveats and nuanced explanations, the resulting number is likely worthless. Reliable metrics should have a clear, straightforward calculation that accurately reflects the quality and status of the TMF. * **End-User Education is Critical for System Success:** The biggest challenge in TMF management is often not system configuration, but end-user education. Consultants must focus on practical application and demonstrating how consistent data entry benefits the user (e.g., using system filters to generate actionable lists), rather than simply demanding compliance. * **Leveraging Digital Standards for Cooperation:** The adoption of standards like the CDISC M11 standardized digital protocol will enable all industry players to speak the same language. This standardization can allow smaller biotech companies to form consortia and cooperate on data sharing, enabling them to compete more effectively with larger organizations. * **The Shift to Digitally Native Documents:** The future of TMF involves moving away from scanning paper or mining documents for data. Systems will become "digitally native," where data points entered into the system will automatically generate the required document narrative, streamlining compliance and ensuring data integrity. * **TMF Champions in Functional Areas:** Companies should identify and empower TMF champions within each functional area (e.g., Data Management, Clinical Monitoring). These champions can speak specifically to the documents their area produces and act as liaisons between their team and the central TMF team, ensuring consistency and context. * **TMF is the Story of the Trial:** The TMF is the complete regulatory record of the trial. If pages are missing or the record is incomplete, the sponsor lacks the full story, jeopardizing regulatory approval and potentially patient safety. ### Tools/Resources Mentioned * **Veeva:** Mentioned as a leading electronic Trial Master File (eTMF) platform. The guest is a Veeva-certified expert. * **ICH GCP E6(R3):** International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, Good Clinical Practice, Revision 3. * **21 CFR Part 11:** FDA regulation concerning electronic records and electronic signatures, relevant for compliant eTMF systems. * **CDISC (Clinical Data Interchange Standards Consortium):** Mentioned in relation to industry standardization efforts. * **M11 Standardized Digital Protocol:** A CDISC initiative aimed at standardizing digital protocols. * **TMF Reference Model (Version 4):** Discussed as the standard moving toward digitally native documents. ### Key Concepts * **Trial Master File (TMF):** The collection of essential documents that individually and collectively permit the reconstruction and evaluation of the conduct of a clinical trial and the quality of the data produced. Referred to as the "heart of the trial." * **eTMF (Electronic Trial Master File):** A digital system used for managing and storing TMF documents, often including audit trails and compliance features. * **Digitally Native Documents:** Documents where the data is captured and stored directly within the system, allowing the system to generate the document narrative, rather than relying on scanned paper or manually uploaded files. * **Sponsor Oversight:** The regulatory responsibility of the trial sponsor (the company funding the trial) to ensure the quality and integrity of the trial, including documentation managed by CROs. * **Audit Trail:** An electronic record that details all actions performed on a document or system entry (who, what, when), which regulators use to verify data integrity and compliance history.

Season 2, Episode 5: The Heart of the TMF with Ann Ackley-Fifer
Heart of the Trial
/@HeartoftheTrial
Sep 1, 2025
This podcast episode provides an in-depth look into the evolution and current state of Trial Master File (TMF) management in clinical research, featuring TMF expert and Veeva-certified consultant Ann Ackley-Fifer. The discussion traces the journey of TMF from its paper-based origins in the early 2000s—where the process was simply "tag it and bag it"—to the complex digital strategies employed today. A core theme is the necessity of foundational TMF knowledge, emphasizing that even the most advanced electronic TMF (eTMF) systems fail without empowered and educated end-users who understand the "why" behind documentation requirements. Ann highlights that her consulting work often pivots from technical system review to focusing on end-user training and experience, ensuring practical application of the technology. A significant portion of the conversation focuses on the impact of regulatory changes, particularly the impending ICH GCP E6 R3 guidelines. The speakers stress that R3 places a much greater emphasis on sponsor oversight and demands increased transparency from Contract Research Organizations (CROs). This transparency is crucial for sponsors to maintain proper control over their clinical trial documentation and data integrity. The shift in regulatory language from "documents" to "records" underscores the industry's move toward digitally native data. The speakers warn that CROs must adapt by offering better oversight tools—such as visibility into in-progress items, issue trends, and quality control metrics—to remain competitive against sponsors who are increasingly bringing eTMF management in-house to gain control. Looking toward the future, the episode explores the potential of industry standardization and cooperation. Ann expresses optimism about the CDISC M11 standardized digital protocol, which aims to ensure all industry players speak the same language and collect uniform data points. This standardization, she argues, will enable greater cooperation, allowing smaller biotech companies to form consortia and compete effectively with larger organizations by sharing data and systems that seamlessly communicate. Furthermore, the discussion touches on the concept of "digitally native documents," where data in the system automatically generates a document narrative, reversing the current process of mining documents for data. However, a major concern raised is the potential loss of foundational TMF knowledge among new entrants, as entry-level roles become increasingly automated by AI and machine learning, risking a lack of understanding regarding the context and patient impact (the "why") of the documentation. Key Takeaways: • **TMF is the Heart of the Trial:** TMF documentation is not a low-priority administrative task; it is the comprehensive, auditable story of the trial from beginning to end, and its integrity directly impacts regulatory approval and patient safety. • **E6 R3 Mandates Sponsor Oversight and CRO Transparency:** The new ICH GCP guidelines empower sponsors to demand greater visibility into the TMF, requiring CROs to provide robust oversight tools and metrics that clearly show the status of documents, quality control, and issue trends. • **Inspection Readiness is a Mindset, Not an Activity:** TMF management must be a continuous process, not a quarterly or semi-annual cleanup effort; regulators look for signs of last-minute QC pushes (e.g., a large influx of QC activity just before an inspection) and view this as a major red flag. • **Veeva Certification Requires Continuous Learning:** Maintaining expertise in platforms like Veeva requires ongoing annual testing and updates to stay current with new features and modules, highlighting the need for specialized, up-to-date system configuration knowledge. • **The Importance of End-User Education:** Technology alone is insufficient; most TMF problems stem from end-user misunderstanding or lack of practical training on how the eTMF system works and how it benefits their specific functional area (the "aha moment"). • **Beware of Worthless Metrics:** If metrics used by a CRO require extensive caveats, nuances, and complicated explanations to calculate, they are likely not providing straightforward, actionable insights for sponsor oversight. • **The Shift to Digitally Native Documents:** Future standards (like CDISC M11) are moving toward systems where data points are standardized and documents are generated from that data, requiring a fundamental shift in how documentation is managed and utilized. • **Risk of Losing Foundational Knowledge:** The increasing automation of entry-level TMF tasks via AI and machine learning risks eliminating the opportunities for new professionals to gain the detective-like, ground-up understanding of regulatory documents and their context. • **Cooperation Drives Competition:** Industry standardization (like CDISC) will facilitate cooperation among smaller biotech companies, allowing them to leverage shared data standards and systems to compete more effectively with large pharmaceutical organizations. • **Identify TMF Champions in Functional Areas:** Companies should designate TMF champions within each functional area (e.g., data management, clinical operations) to bridge the gap between specialized TMF teams and the document producers, ensuring context-specific compliance. • **Audit Trails Tattle:** Regulators will use system audit trails to identify inconsistencies, such as a lead data manager not logging in for months, or a massive backlog of documents being QCed immediately prior to an inspection. Tools/Resources Mentioned: * **Veeva:** Mentioned as a leading electronic Trial Master File (eTMF) platform. * **ICH GCP E6 R3:** The latest version of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines for Good Clinical Practice. * **CDISC M11 Standard:** The standardized digital protocol being developed by the Clinical Data Interchange Standards Consortium (CDISC). * **TMF Reference Model (Version 4):** A reference model being updated to address digitally native documents. Key Concepts: * **Trial Master File (TMF):** The collection of essential documents that individually and collectively permit the evaluation of the conduct of a clinical trial and the quality of the data produced. * **Electronic TMF (eTMF):** A digital system used to manage the TMF, often required to be 21 CFR Part 11 compliant. * **Digitally Native Documents:** Documents or records where the data originates and resides within a structured digital system, rather than being digitized from paper or unstructured files. * **Sponsor Oversight:** The regulatory requirement for the pharmaceutical company sponsoring the trial to maintain control and visibility over the activities delegated to CROs. * **21 CFR Part 11:** FDA regulations governing electronic records and electronic signatures, ensuring data integrity and security.

My Take on Veeva's Strong Q2 Financials
Corporate Decoder
/@CorporateDecoder
Aug 30, 2025
This video provides a financial analysis of Veeva Systems' second quarter fiscal 2026 results, highlighting strong performance across key metrics. The speaker details Veeva's revenue growth, profitability, and cash flow, positioning the company as a robust cloud-based software provider for the life sciences industry. The analysis delves into the breakdown of revenue streams, noting significant growth in customer services, and discusses the implications of these numbers for Veeva's market position and operational efficiency. Key Takeaways: * **Veeva's Strong Financial Health:** Veeva Systems reported impressive Q2 fiscal 2026 results, with total revenue increasing 15% year-over-year to $779 million, operating income up 20%, and net income surging 25%, demonstrating robust growth and profitability. * **Customer Services Driving Engagement:** Customer services revenue saw a substantial 21% year-over-year increase, outpacing the 12% growth in subscription revenue. This indicates a deepening relationship with existing clients, suggesting increased adoption of additional support, consulting, and expanded use of Veeva's platforms. * **Operational Efficiency and Leverage:** The faster growth in operating income (20%) and net income (25%) compared to total revenue (15%) signifies that Veeva is effectively managing its costs and achieving operational leverage, translating top-line growth into even stronger bottom-line results. * **Stable and Growing Ecosystem:** Veeva's consistent growth and specialization in the life sciences industry create a stable and expanding ecosystem. Its strong financial trajectory underscores the ongoing demand for specialized software and services within the pharmaceutical and biotech sectors.

Veeva Systems Q2 Earnings Breakdown: AI & IQVIA Deal Power Growth
TalkTickers: AI Podcast Discussing Earnings Calls
/@TalkTickersPodcast
Aug 30, 2025
This video provides an in-depth analysis of Veeva Systems' Q2 2026 earnings, arguing that the market's negative reaction to a short-term GAAP earnings miss is fundamentally misguided. The core thesis presented is that three massive strategic catalysts—the resolution of the IQVIA dispute, the ambitious "Agentic AI" roadmap, and dominant execution in the next-generation CRM market—are being severely undervalued, positioning Veeva for significant long-term growth within the life sciences sector. The analysis details two major strategic shifts. First, the landmark partnership with IQVIA resolves a decade-long legal and data access conflict that the CEO previously characterized as a major structural weakness. This deal transforms a competitor into an ally, enabling Veeva to integrate IQVIA's crucial, top-tier datasets into its platform offerings (like Network and Nitro). This integration fundamentally derisks Veeva’s entire commercial business, removes a significant objection faced by its global sales teams, and allows Veeva to offer a more complete, wholesome solution to biopharma clients. Second, the video highlights Veeva’s long-term commitment to "Agentic AI." This is defined not as simple feature additions, but as the creation of super-smart digital assistants designed to automate complex, multi-step workflows across the life sciences value chain. Veeva asserts it holds a "structural advantage" because its software already serves as the industry's operational backbone, allowing it to deeply integrate these AI agents directly into existing workflows—a capability generic AI companies lack. The roadmap is concrete and phased: the first AI agents are scheduled for deployment in the CRM platform in December 2025, followed by subsequent waves targeting clinical, regulatory, and safety domains through 2027. Finally, the video underscores Veeva’s strong current execution, particularly in the competitive battle for the next-generation CRM market. Veeva’s Vault CRM has secured 9 of the top 20 biopharma giants, compared to only 3 for its main competitor, Salesforce. More critically, Veeva has already achieved successful customer go-lives, demonstrating a massive execution gap, as the competitor’s earliest similar customer deployment is not anticipated until late 2026. This execution prowess, combined with the strategic data and AI initiatives, leads the analysts to conclude that Veeva is an "outperform" stock pick, asserting that the market is focusing on short-term noise while missing the long-term value creation. Key Takeaways: * **Veeva's AI Strategy is Workflow-Centric:** Veeva is investing heavily in "Agentic AI"—specialized, multi-step digital assistants designed to automate complex tasks within life sciences workflows (CRM, clinical, regulatory, safety). This validates the industry trend toward highly integrated, domain-specific AI solutions rather than generic LLMs. * **Structural Advantage in AI Integration:** Veeva’s competitive edge in AI is based on its deep integration as the existing workflow backbone for the life sciences industry. Consulting firms like IntuitionLabs must leverage their own deep industry knowledge and existing integration capabilities (especially around Veeva platforms) to create custom AI solutions that capitalize on this structural advantage. * **Agentic AI Roadmap Confirms Future Focus:** The phased rollout of AI agents—starting with CRM in December 2025, followed by clinical, regulatory, and safety through 2027—provides a clear signal regarding Veeva’s strategic priorities. IntuitionLabs should align its custom AI/LLM development (e.g., Medical Info Chatbots, Sales Ops Assistants) to complement or integrate with these specific platform expansions. * **IQVIA Deal Enhances Commercial Data Strategy:** The resolution of the decade-long IQVIA data dispute removes a major barrier, allowing Veeva to integrate crucial datasets into products like Network and Nitro. IntuitionLabs should anticipate increased client demand for data engineering and integration services focused on leveraging combined IQVIA and Veeva data assets for commercial intelligence. * **Vault CRM Dominance is Confirmed:** Veeva has secured 9 of the top 20 biopharma companies for its new Vault CRM, establishing it as the dominant platform for future commercial operations in the largest pharmaceutical enterprises. This confirms Vault CRM consulting as a high-growth service area. * **Execution Gap Creates Opportunity:** The fact that Veeva has major Vault CRM customers live while competitors lag significantly (with projected go-lives not until late 2026) highlights the critical importance of successful, rapid implementation. IntuitionLabs must emphasize speed and expertise in its Veeva CRM consulting services to capitalize on this competitive execution gap. * **Regulatory and Clinical AI is a Priority:** The explicit mention of AI agents for regulatory and safety domains confirms Veeva’s commitment to automating compliance and quality processes. This reinforces the need for IntuitionLabs to prioritize developing AI solutions that streamline compliance tracking, audit trails, and GxP requirements. * **Market Focus on Long-Term Value:** The video argues that the market is obsessing over short-term financial metrics (GAAP miss) while ignoring massive strategic catalysts (IQVIA, AI). This reinforces the need for IntuitionLabs to communicate the long-term strategic value of its AI and compliance solutions to clients, focusing on derisking operations and unlocking new commercial opportunities. Tools/Resources Mentioned: * Veeva Systems (Vault CRM, Network, Nitro) * IQVIA (Data giant and strategic partner) * Salesforce (Main competitor in the CRM space) Key Concepts: * **Agentic AI:** A term used by Veeva to describe highly specialized, multi-step digital assistants or agents capable of handling complex jobs autonomously, trained specifically on life sciences workflows. * **Structural Advantage:** Veeva’s competitive edge derived from its existing position as the foundational software platform (the "backbone") where life sciences work is already performed, allowing for deep, seamless AI integration. * **Vault CRM:** Veeva’s next-generation Customer Relationship Management platform, built on the Vault architecture, which is rapidly gaining adoption among top biopharma companies.

Veeva Systems Stock Price Target Surges Amid Positive Analyst Ratings
NewsBOT Business
/@NewsBOTBusiness
Aug 30, 2025
This video provides a detailed financial analysis of Veeva Systems (NYSE:VEEV), focusing on recent positive adjustments to stock price targets by major financial analysts, reflecting a strong and optimistic outlook on the company's performance within the cloud-based software sector for the life sciences industry. The analysis establishes that despite some cautious ratings, the overall market sentiment is bullish, driven by impressive recent earnings and Veeva’s sustained position as a market leader providing essential solutions to pharmaceutical and biotechnology firms. The core of the analysis centers on the consensus among financial institutions. While Morgan Stanley raised its target from $210 to $222, it maintained an "underweight" rating, suggesting caution. However, this cautious view is offset by significantly more optimistic ratings from other firms: Mizuho raised its objective from $280 to $295 with an "outperform" rating, and BTIG increased its target from $335 to $340 with a "buy" rating. Barclays and Evercore ISI also adjusted targets upward, leading to a strong consensus rating of "Moderate Buy" among analysts, with an average target price settling near $299.88. This broad agreement highlights the perceived stability and growth potential of Veeva’s regulated enterprise software platforms. This bullish sentiment is strongly supported by Veeva’s recent financial disclosures. The company reported earnings per share (EPS) of $1.97 for the last quarter, significantly surpassing the consensus estimate of $1.74 by 23%. Furthermore, quarterly revenue reached $759.04 million, exceeding analyst expectations of $728.38 million. This financial performance marks a substantial 16.7% increase in revenue compared to the same period last year, demonstrating robust growth in a competitive market. Financially, Veeva maintains excellent health, evidenced by a net margin of 27.29% and a return on equity of 14.19%. Looking forward, analysts predict Veeva Systems will post an EPS of $4.35 for the current fiscal year, reinforcing expectations of continued expansion. The video notes that Veeva continues to innovate and expand its product offerings, specifically mentioning Veeva Commercial Cloud and Veeva Vault. These platforms are crucial for enabling pharmaceutical and biotech firms to enhance operations and improve customer engagement while navigating complex regulatory environments. The growing interest from institutional investors, alongside the positive analyst momentum, suggests that Veeva is well-positioned for sustained growth, confirming its dominance in providing compliant, cloud-based solutions tailored for the highly regulated life sciences sector. Key Takeaways: * **Veeva’s Market Dominance is Validated:** The strong consensus among analysts, resulting in a "Moderate Buy" rating and an average price target of nearly $300, confirms Veeva’s secure position as the indispensable technology provider for life sciences commercial and clinical operations. * **Significant Revenue Growth Signals Market Expansion:** Veeva’s 16.7% year-over-year revenue increase and the $759.04 million quarterly revenue beat indicate that the life sciences sector is rapidly adopting and expanding its use of Veeva platforms, creating an expanding addressable market for specialized consulting and integration services. * **High EPS Beat Reflects Effective Monetization:** The substantial beat on EPS ($1.97 actual vs. $1.74 estimated) suggests that clients are not only adopting Veeva but are also successfully implementing and leveraging the platform’s high-value features, often requiring expert guidance in areas like AI integration and complex system customization. * **Focus on Core Products:** The explicit mention of Veeva Commercial Cloud and Veeva Vault reinforces that these product lines are the primary drivers of Veeva’s growth, signaling where consulting firms should concentrate their expertise and custom AI development efforts. * **Financial Health Guarantees Platform Stability:** Veeva’s robust financial metrics (27.29% net margin and 14.19% ROE) ensure sustained investment in R&D, meaning the platform will continue to evolve rapidly, necessitating continuous training and specialized consulting for clients to keep pace with new features and regulatory updates. * **Institutional Confidence in Life Sciences Tech:** The acquisition of stakes by institutional firms like Trust Company of Toledo and Abound Financial highlights that major investors view the regulated life sciences cloud sector as a stable, high-growth area, validating the strategic decision to specialize in this niche. * **Anticipated Complexity Requires Expertise:** The predicted EPS of $4.35 for the current fiscal year implies continued product complexity and feature rollouts, increasing the demand for expert Veeva CRM consultants and AI developers capable of integrating advanced solutions (like LLMs) into the core Veeva architecture. * **Strategic Alignment with Regulatory Compliance:** As Veeva continues to solidify its role in providing solutions that enable pharmaceutical and biotech firms to enhance operations, the need for partners who can ensure these enhancements remain compliant with FDA and GxP standards (a core offering of IntuitionLabs.ai) will intensify. Tools/Resources Mentioned: * Veeva Systems (NYSE:VEEV) * Veeva Commercial Cloud * Veeva Vault Key Concepts: * **Price Target:** A financial analyst’s estimate of the future price of a stock, used to guide investment decisions. * **Underweight Rating:** An analyst rating suggesting that the stock is expected to perform worse than the average stock in its sector or the overall market. * **Outperform/Buy Rating:** Analyst ratings suggesting the stock is expected to perform better than the market or is a strong investment opportunity. * **Earnings Per Share (EPS):** A company's net profit divided by the number of outstanding common shares, a key indicator of profitability. * **Net Margin:** The percentage of revenue left after all operating expenses, interest, taxes, and preferred stock dividends have been deducted, indicating financial efficiency.

Veeva Systems Q2: IQVIA, AI & CRM Wins vs. Market Expectations
TalkTickers: AI Podcast Discussing Earnings Calls
/@TalkTickersPodcast
Aug 29, 2025
This video provides an in-depth analysis of Veeva Systems' Fiscal Q2 2026 earnings call, dissecting the life sciences software giant's financial performance, strategic initiatives, and market reception. The discussion highlights Veeva's strong underlying revenue and non-GAAP EPS beats, which were paradoxically met with a stock dip due to a GAAP EPS miss, underscoring intense investor scrutiny and high expectations within the current market. The hosts meticulously break down the numbers, management's outlook, and the significant strategic developments that are shaping Veeva's future in the pharmaceutical and biotech sectors. A major focus of the earnings call, and subsequently the podcast, was the "transformative" IQVIA settlement. This resolution of a decade-long legal dispute eliminates critical data-use restrictions that had previously hampered Veeva's commercial and clinical offerings, particularly for products like Veeva Network, Nitro, and Veeva EDC. This strategic shift turns a former rival into a collaborator, allowing for seamless data integration and positioning IQVIA as a Contract Research Organization (CRO) partner for Veeva's clinical trial software. This foundational change is expected to significantly strengthen Veeva's long-term market position, making its ecosystem stickier and harder for competitors to penetrate. Furthermore, the video delves into Veeva's ambitious "agentic AI" strategy, which involves AIs designed to actively perform tasks, interact with systems, and make decisions. The debut of these AI agents is planned for December within Vault CRM and commercial content applications, with a phased rollout across clinical, regulatory, safety, quality, and clinical data agents through 2027. While no material revenue contribution is expected in the immediate fiscal years, Veeva aims to create billions in industry value through automated workflows and a fundamental shift in user interaction. The company believes its "structural advantage"—its deep industry footprint and existing 50+ specific applications as systems of record—gives it a unique right to win in life sciences AI. The podcast also examines the accelerating momentum of Vault CRM, which now boasts over 100 live customers and commitments from nine of the top 20 global biopharmas, rapidly gaining ground against Salesforce with faster implementation speeds. Key Takeaways: * **Veeva's Q2 Financials and Market Disconnect:** Despite reporting strong revenue ($789.1 million, up 17% YoY) and non-GAAP EPS ($1.99) beats, Veeva's stock dipped by 3.7% after hours due to a GAAP EPS miss of $1.11. This illustrates high investor expectations and sensitivity to earnings quality, even when underlying operational performance is robust and guidance is raised. * **Transformative IQVIA Settlement:** The resolution of a decade-long legal battle with IQVIA is a "hinge event," eliminating data-use restrictions that previously limited Veeva Network and Nitro. This allows for seamless data integration, enhances Veeva EDC by positioning IQVIA as a CRO partner, and significantly strengthens Veeva's commercial and clinical cloud offerings. * **Ambitious "Agentic AI" Rollout:** Veeva plans to launch its first "agentic AI" solutions in December, starting with Vault CRM and commercial content applications. This will be followed by a phased release of agents for clinical, regulatory, safety, quality (2026), and clinical data (2027), aiming for a fundamental shift in user interaction and workflow automation. * **Long-Term AI Value Creation, Not Immediate Revenue:** While Veeva anticipates its AI strategy to create billions of dollars in value for the industry by boosting efficiency, no material revenue contribution is expected in fiscal 2026 or 2027. The focus is on early adoption and proving value, indicating a long-term strategic play. * **Veeva's "Structural Advantage" in AI:** Veeva believes its deep industry footprint, with around 50 existing industry-specific applications serving as systems of record, provides a "structural advantage" for its AI strategy. This allows them to embed AI directly into existing workflows, offering integrated solutions rather than standalone tools. * **Vault CRM Gaining Significant Traction Against Salesforce:** Vault CRM now has over 100 live customers, including commitments from nine of the top 20 global biopharmas, with two already live in major markets. This is presented as a significant lead over Salesforce, which reportedly has only three top 20 verbal commitments. * **Faster Implementation as a Key Differentiator:** Veeva highlights its ability to bring top 20 pharma customers live in major markets in under two years for Vault CRM, contrasting with Salesforce's projected longer timelines (late 2026 for a single region, potentially 2029 for global implementation). * **Expanded Focus on Quality Cloud:** Veeva is elevating its internal focus on Quality Cloud, expanding its offerings to include Laboratory Information Management Systems (LIMS), batch release, and validation management, aiming to build a comprehensive quality suite. * **Supportive Macro Environment:** Veeva benefits from positive industry tailwinds, including rising large pharma R&D spending and accelerating global medicine spending. The potential for AI to shorten drug development cycles further supports a stable environment for Veeva's customers. * **Strategic Transformation to a Comprehensive Platform:** The Q2 results underscore Veeva's successful multi-year transformation from solely a CRM provider to a comprehensive platform, data, and services partner for the entire life sciences industry, with the IQVIA deal and AI strategy being central to this evolution. * **Execution Risk in Large Migrations:** While the long-term strategic narrative is compelling, investors will closely monitor Veeva's execution of large Vault CRM migrations over the next couple of years (fiscal 2026-2027) as a key execution risk. **Tools/Resources Mentioned:** * **Veeva Network:** A data solution for managing healthcare professional data. * **Veeva Nitro:** A commercial data warehouse solution. * **Veeva EDC:** Electronic Data Capture for clinical trials. * **Vault CRM:** Veeva's customer relationship management platform for life sciences. * **Quality Cloud:** Veeva's suite of quality management applications, expanding into LIMS, batch release, and validation management. * **Crossix Audiences:** Veeva's data analytics segment, particularly its usage-based component. * **Compass Prescribers:** A Crossix product for measuring marketing effectiveness for healthcare providers. * **SAP, Workday:** Mentioned as large enterprise systems with which Veeva's AI agents will interoperate. **Key Concepts:** * **Agentic AI:** Refers to AI systems designed to not just process information but to actively perform tasks, interact with other systems, and make decisions, thereby automating complex workflows. * **Structural Advantage:** Veeva's term for its competitive edge in AI, stemming from its existing deep integration within the life sciences industry through over 50 specialized applications that serve as systems of record, allowing AI to be embedded directly into established workflows. * **Horizontal Software:** Veeva's ambition to expand its software offerings beyond the life sciences industry, initially using its CRM platform as a base for broader market applications.

Season 1 Episode 1: Building the Right Data and Technology Foundation for Safety
Veeva Systems Inc
@VeevaSystems
Aug 29, 2025
This video, from the Veeva podcast "Safety Revolution," features David Kološić (Veeva) and Aniket Agarwal (Director for Data Operations and Analytics in Patient Safety at Sandoz), discussing the critical role of a robust data and technology foundation in pharmacovigilance (PV). The conversation centers on how Sandoz, as a large generic and biosimilar company, is navigating its growth while enhancing operational efficiency and speed in patient safety through strategic technology adoption. The discussion highlights Sandoz's deliberate shift from a historical landscape of "best-in-class" siloed systems to a platform-based approach. This transformation is driven by the need to overcome challenges associated with maintaining complex integrations between disparate systems and to ensure sustainable growth without a proportional increase in operational teams. Aniket explains that while other domains like clinical and regulatory have adopted platforms earlier, PV's slower pace is due to stringent regulations, frequent inspections, and the necessity of maintaining data compatibility for products with long market lifecycles (30-40 years). The core idea is to harmonize data and technology across global development (clinical, regulatory, safety) to establish a single source of truth, reducing manual reconciliation and improving data quality. A significant portion of the conversation is dedicated to the strategic application of automation and AI in PV. Aniket advocates for a "grounded approach," emphasizing that organizations should first identify specific problems and leverage simpler automation for quick efficiencies before deploying more complex AI solutions. He identifies high-impact AI use cases, particularly in the Individual Case Safety Report (ICSR) space, such as ingesting unstructured data (e.g., non-E2B reports which constitute a significant portion of incoming data) and generating human-readable narratives. Beyond ICSRs, AI is seen as transformative for moving from traditional to predictive signal detection, enhancing the quality of detected signals. The speakers also touch upon the balance between making reporting easy for healthcare professionals and patients (e.g., supporting regional languages) and the need for technology to structure this diverse intake downstream. The ultimate vision for 2030 is "no-touch" end-to-end case processing, with AI solving the remaining 40% of complex scenarios, contingent on evolving regulatory frameworks and building confidence in AI-generated data. Key Takeaways: * **Shift to Platform-Based PV:** Sandoz is moving from siloed, "best-in-class" systems to a unified platform approach to achieve sustainable operations, reduce integration complexities, and ensure systems evolve at a consistent pace. This is crucial for long-term efficiency and growth in pharmacovigilance. * **Drivers for PV Platform Adoption:** The need for harmonization of data and technology across global development (clinical, regulatory, safety) is a prime driver. A platform approach simplifies data flow, reduces maintenance, and supports cross-functional collaboration. * **Challenges in PV Technology Evolution:** PV has been slower to adopt platform solutions due to strict regulations, frequent inspections, the need for data compatibility for products with decades-long market presence, and the inherent risk associated with system changes. * **Importance of Data Standardization:** Standardizing data across regulatory, clinical, and safety domains is critical for establishing a "one source of truth," reducing manual reconciliation efforts, and improving the efficiency and quality of reporting (e.g., for PSURs/DSURs). * **Overcoming Data Silos and Mindset Shifts:** Achieving data standardization requires breaking down historical departmental silos and fostering a mindset shift towards common organizational goals, even if it involves an iterative process and governance to align definitions. * **Grounded Approach to Automation and AI:** Prioritize solving specific problems with simpler automation for quick wins and agility. Reserve AI for more complex, high-impact use cases where traditional automation is insufficient, adopting a phased approach if necessary. * **High-Impact AI Use Cases in PV:** Key areas where AI can drive significant value include ingesting and structuring unstructured incoming data (e.g., non-E2B reports), generating advanced, human-readable narratives for ICSRs, and transitioning from traditional to predictive signal detection. * **Balancing Reporting Ease and Data Structure:** To encourage higher reporting rates, it's essential to make the reporting process as simple as possible for users (e.g., supporting regional languages, flexible input formats). Technology, particularly AI, can then be leveraged downstream to decipher and structure this diverse information. * **Benefits of Cross-System Analytics:** A platform approach enables efficient, real-time cross-system analytics for periodic reports, reducing manual data extraction and reconciliation, and ensuring consistent information for regulatory submissions and inspections. * **Regulatory Harmonization and Frameworks:** Organizations like ICH, EMA, and FDA are actively working on frameworks and guidance to support the adoption of automation and AI in PV, indicating a growing openness and a shared goal with the industry towards safer and more efficient processes. * **Vision for "No-Touch" PV:** The aspirational goal for 2030 is end-to-end automated (no-touch) case processing, where systems can consistently handle a vast majority of scenarios, driving both quality and efficiency, and freeing up resources for more complex tasks. * **Building Regulator Confidence in AI:** As AI technologies advance, it's crucial to collaborate with regulators to understand their expectations, address concerns like hallucination, and ensure AI-generated data remains usable and fit for purpose within a compliant framework. **Tools/Resources Mentioned:** * **Veeva:** A leading platform provider in the pharmaceutical industry, hosting the podcast. * **E2B:** An international standard for the electronic transmission of individual case safety reports (ICSRs). * **ICH (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use):** Mentioned as an organization striving for harmonization. * **EMA (European Medicines Agency):** Referenced for its efforts in standardization (e.g., through UdraVigilance) and guidance on AI. * **FDA (U.S. Food and Drug Administration):** Referenced for its guidance on AI. * **CIOMS (Council for International Organizations of Medical Sciences):** Mentioned as producing material on AI. **Key Concepts:** * **Pharmacovigilance (PV):** The science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drug-related problem. * **Individual Case Safety Report (ICSR):** A report detailing a single suspected adverse drug reaction in a patient. * **Periodic Safety Update Report (PSUR):** A periodic report providing an update on the worldwide safety experience of a medicinal product. * **Development Safety Update Report (DSUR):** A periodic report providing an update on the worldwide safety experience of an investigational medicinal product. * **Signal Detection:** The process of identifying and assessing potential safety signals from various data sources. * **Generics and Biosimilars:** Types of pharmaceutical products Sandoz specializes in. * **GxP (Good x Practice):** A collection of quality guidelines and regulations created to ensure that products are safe and meet their intended use. * **21 CFR Part 11:** Regulations issued by the FDA that set forth the criteria under which electronic records and electronic signatures are considered trustworthy, reliable, and equivalent to paper records.

Tom Broker's 3 Tips To CRUSH Your Q4 Sales Goals
Self-Funded
@SelfFunded
Aug 29, 2025
This satirical video, presented by the persona "Tom Brokers," offers a cynical and aggressive perspective on how sales professionals, particularly those in the brokerage or high-stakes commercial sector, are pressured to meet and exceed their quotas during the critical fourth quarter (Q4). The video frames Q4 not as a time for winding down or maintaining client relationships, but as a period of intense, singular focus on new revenue generation and commission maximization, often at the expense of long-term client service and ethical practice. The core methodology presented is a three-pronged approach designed to maximize immediate sales volume and personal margin. The first, and most foundational, tip is the complete abandonment of existing clients—referred to as "straight up ghosting." The rationale is that every minute spent addressing current client service issues, such as explaining deductibles or managing existing contracts, is a minute diverted from closing new business. This strategy effectively pushes all service obligations and potential client dissatisfaction into the next fiscal year, prioritizing short-term quota achievement over client retention and satisfaction, highlighting the chaotic nature of misaligned sales incentives. The second and third tips focus on exploiting market conditions and competitor weaknesses during the critical renewal season. Tip two advises against fighting or negotiating carrier-mandated rate increases (cited as 30%), instead recommending that the broker simply forward the increase to the client and use "inflation" as a universal, unchallengeable excuse. This tactic shields the broker from accountability while shifting blame to external economic factors, such as tariffs, supply chains, or even historical events like the Suez Canal blockage. This strategy allows the broker to maintain their relationship with the carrier while avoiding difficult value-justification conversations with the client. Finally, the third tip leverages the anticipated service vacuum created by competitors who are likely following Tip Number One. By positioning themselves as a "knight in shining armor," the aggressive broker swoops in to "save" neglected customers. This late-year opportunity is used not just to acquire new clients, but specifically to sell high-margin, complex, or ancillary products—such as GAP plans, Icas (Individual Coverage Health Reimbursement Arrangements), and "tax loophole wellness products"—that may be poorly understood but generate substantial commission. The ultimate goal is to "maximize them margins," reinforcing the video's theme that Q4 is purely about personal financial gain, regardless of the complexity or long-term suitability of the products sold. Key Takeaways: • **The Peril of Q4 Quota Pressure:** The video highlights how intense, short-term sales pressure in Q4 can lead to severely misaligned incentives, causing sales professionals to abandon fundamental business practices like client service and relationship management in favor of immediate revenue capture. • **Acquisition Over Retention Strategy:** The satirical advice to "ghost" existing clients underscores a common, yet detrimental, sales pitfall: prioritizing the closing of new business over nurturing the current customer base, which is crucial for long-term recurring revenue and operational stability. • **Leveraging Renewal Cycles for Opportunity:** Renewal season is presented as a high-stakes period where large rate increases (e.g., 30%) create both risk and opportunity; the strategy is to use external factors (inflation, supply chain) as a shield to avoid responsibility for price hikes. • **The Value of Competitor Service Gaps:** A critical insight is that poor service delivery by competitors creates immediate, high-conversion sales opportunities late in the year, allowing aggressive sales teams to position themselves as rescuers rather than mere vendors. • **Maximizing Ancillary Product Sales:** The focus shifts from core offerings to high-margin, complex products (GAP plans, Icas, wellness products) that are often easier to push during a time of client distress, generating higher commissions even if their value proposition is opaque. • **The Role of Excuse-Making in Sales:** The concept of "advanced excusemaking" (blaming inflation, tariffs, etc.) demonstrates the need for sales teams to have ready-made justifications for market volatility, though in a professional context, this translates to robust value justification and proactive communication. • **Misaligned Incentives and Client Outcomes:** The skit serves as a warning about the consequences of commission structures that heavily reward new sales volume without balancing metrics for client satisfaction, retention, or long-term relationship health. • **Time Management and Focus:** The core time management principle, though exaggerated, is that sales reps must ruthlessly prioritize activities that lead directly to closing new deals, viewing service tasks as distractions during peak sales periods. • **The "Hero" Positioning:** By capitalizing on competitor neglect, the sales professional can bypass the typical sales process and adopt a "hero" or "consultant" persona, which often reduces client scrutiny regarding commission rates or product complexity. Key Concepts: * **Ghosting:** The complete cessation of communication or service delivery to existing clients to free up time for new sales acquisition. * **Renewal Season:** The critical period, typically late in the year, where existing client contracts are renegotiated, often involving significant rate changes, creating high churn potential and new sales opportunities. * **Margin Maximization:** The strategy of focusing sales efforts specifically on products or services that yield the highest personal commission or profit percentage, often prioritizing these over the client’s optimal solution. * **Advanced Excusemaking:** A satirical term for developing and deploying external, non-negotiable reasons (like inflation) to justify unfavorable market conditions or price increases to clients.

Veeva Systems Stock Soars: Analysts Boost Price Targets Amid Strong Earnings
NewsBOT Business
/@NewsBOTBusiness
Aug 29, 2025
This video provides a comprehensive financial analysis of Veeva Systems (NYSE: VEEV) following a robust earnings report that significantly surpassed market expectations. The central focus is the resulting surge in analyst confidence, leading to multiple upward revisions of stock price targets. The analysis establishes that Veeva's strong financial performance validates its continued dominance and strategic importance within the life sciences technology sector, particularly concerning regulated enterprise software and cloud solutions. The analysis details the impressive financial metrics that fueled the market excitement. Veeva reported an Earnings Per Share (EPS) of $1.97 for the quarter, which exceeded the analysts' consensus estimate of $1.74 by a notable 23%. Furthermore, the company’s quarterly revenue reached $759.04 million, significantly outpacing the anticipated $728.38 million. This revenue figure marks a substantial 16.7% increase compared to the same quarter in the previous year, demonstrating accelerated growth and successful execution across its product lines. This financial strength prompted a flurry of positive assessments from major financial institutions. Wells Fargo and Company raised its target from $300 to $326, assigning an "overweight" rating. Other significant target increases included Evercore ISI (from $285 to $295), Truest Financial (from $217 to $230), and UBS Group (from $250 to $285). The overall market consensus, based on insights from 16 buy ratings, seven holds, and two sells, stands at a "moderate buy" with an average target price of $298.68, reflecting widespread confidence in Veeva’s future performance and growth trajectory. The video also touches upon the market structure supporting Veeva, noting strong institutional ownership currently standing at 88.2%, with several large financial entities recently establishing new positions. Crucially for the life sciences industry, the analysis reinforces Veeva’s core value proposition: providing cloud-based software solutions tailored for the sector, specifically citing key offerings such as Veeva Commercial Cloud (for Customer Relationship Management, or CRM) and Veeva Vault Promomats (for digital asset management). The positive outlook is further cemented by analysts forecasting an EPS of $4.35 for the current fiscal year, suggesting sustained momentum and platform expansion. ### Key Takeaways * **Validation of Veeva Ecosystem Investment:** Veeva’s robust financial performance and 16.7% year-over-year revenue growth validate the strategic decision by pharmaceutical and life sciences companies to standardize on the Veeva platform, confirming the long-term viability and necessity of specialized Veeva consulting services. * **Growing Demand for Implementation Partners:** The significant revenue beat and growth indicate expanding adoption and deeper integration of Veeva products (Commercial Cloud, Vault) across the target market, creating a growing demand for expert partners capable of complex implementation, customization, and system optimization. * **Focus Areas for Commercial Operations:** Specific mention of Veeva Commercial Cloud (CRM) and Veeva Vault Promomats highlights the areas where clients are currently directing substantial investment, providing clear focus areas for AI and consulting services aimed at enhancing commercial workflows and digital asset compliance. * **High Analyst Confidence Signals Stability:** The consensus "Moderate Buy" rating and the average target price of $298.68 offer strong reassurance to potential clients that their investment in the Veeva platform is supported by a financially stable and growing vendor, mitigating platform risk. * **Opportunity for AI Integration in Regulated Workflows:** Veeva’s outperformance in the regulated software space reinforces the need for technology solutions that maintain compliance. This creates a prime opportunity for firms specializing in integrating AI and LLMs into Veeva workflows while adhering to GxP and 21 CFR Part 11 requirements. * **Institutional Support for Long-Term Dominance:** High institutional ownership (88.2%) suggests that major financial entities view Veeva as a long-term leader in the life sciences technology space, confirming that specialization in the Veeva ecosystem is a strategically sound, low-risk business decision. * **Minimal Insider Selling Impact:** While some insider trading activity was noted (two directors selling shares), the transactions were minor relative to their retained holdings, which collectively still represent 10.3% of the company’s stock, indicating that the sales were likely routine portfolio management rather than a sign of internal concern. * **Future Platform Expansion Expected:** The forecast of $4.35 EPS for the current fiscal year implies continued investment in product development and R&D by Veeva, which will inevitably introduce new features, modules, and integration points that require specialized consulting expertise for client adoption. ### Tools/Resources Mentioned * **Veeva Systems (NYSE: VEEV):** The primary subject of the financial analysis. * **Veeva Commercial Cloud:** Cloud-based software suite focused on customer relationship management (CRM) for the life sciences industry. * **Veeva Vault Promomats:** A specific Veeva Vault application used for digital asset management and ensuring regulatory compliance of promotional materials. * **MarketBeat.com:** Cited as the source for the consensus analyst rating and average price target. ### Key Concepts * **EPS (Earnings Per Share):** A company's net profit divided by the number of outstanding shares of its common stock; used as an indicator of profitability. Veeva's reported EPS of $1.97 significantly beat estimates. * **Overweight Rating:** An analyst recommendation suggesting that a stock is expected to outperform the average return of the stocks in the analyst's coverage universe or the relevant benchmark index. * **Institutional Ownership:** The percentage of a company's stock held by large financial organizations, such as mutual funds, pension funds, and endowments. High institutional ownership (88.2% for Veeva) often indicates strong market confidence. * **Digital Asset Management:** The process of organizing, storing, and retrieving digital assets (like marketing materials or regulatory documents), a key function provided by Veeva Vault Promomats in a regulated environment.

Veeva Systems Q2 2026 Earnings Call | Q2 2026 Earnings Conference Call | Q2 2026 Results
Investing 101
/@i101_in
Aug 28, 2025
This earnings call provides an in-depth strategic update on Veeva Systems' performance in Q2 Fiscal Year 2026, centering on the advancement of its "Industry Cloud Vision" which integrates industry-specific software, data, and business consulting. CEO Peter Gastner highlighted two major transformative developments: the rapid progress of Veeva AI and the resolution of the decade-long legal dispute with IQVIA. The discussion emphasized that these moves are designed to remove structural limitations, particularly in the Commercial Cloud, and significantly expand Veeva's total addressable market through innovative, deeply embedded technology solutions. The company reported strong financial results ahead of guidance, with total revenue of $789 million and non-GAAP operating income of $353 million, driven by broad-based execution across both R&D and Commercial segments. A central theme was the strategic liberation of the Commercial Cloud. The resolution with IQVIA removes a critical barrier, allowing Veeva to integrate industry-leading IQVIA data directly into its commercial products like Veeva Network, Veeva Nitro (commercial analytics), and other offerings. This integration was previously impractical, creating a "hole in the boat" for these products. This resolution, combined with the earlier migration of CRM off the Salesforce OEM platform to Vault CRM, means the Commercial Cloud now has "no limits," mirroring the integrated maturity and growth potential previously seen primarily in the Development Cloud. Management anticipates this will be transformative, driving broader adoption of the commercial suite—including Campaign Manager, Service Center, and Patient CRM—and increasing the stickiness of their business consulting services. The company detailed its long-term strategy around "Agentic AI," positioning it as the next fundamental phase of the Vault platform, which now incorporates content, data, and intelligent agents. Veeva asserts a "structural advantage" or "right to win" in developing deep, industry-specific agents because its applications are the systems of record, placing the AI directly within the user's workflow and adhering to existing security and transactional rules. While AI is expected to create billions of dollars of value for the industry—through productivity gains in commercial operations and potential reduction of outsourced labor in R&D (e.g., a 50% reduction goal for TMF processing)—management stressed a measured approach to monetization, projecting no material revenue contribution from Veeva AI until FY 2027. Furthermore, the company emphasized that every AI project is inherently a business consulting project, requiring significant change management to redefine human and agent workflows. In terms of execution, the migration to Vault CRM continues to gain momentum, with nine Top 20 pharmaceutical companies now committed, compared to three for Salesforce. Crucially, two Top 20 customers are now live in major markets less than two years after commitment, providing critical proof points for successful implementation. This success is contrasted with the perceived high risk and long timeline (potentially extending to 2029) associated with Salesforce's competitive efforts. R&D Cloud performance remains strong, with particular focus on the elevated stature of Quality Cloud, which is expanding into Laboratory Information Management Systems (LIMS) and batch release products. Finally, the Crossix business continues to be a meaningful driver of growth in commercial, fueled by market share gains and an expanding product footprint, particularly in the high-growth, usage-based Audiences segment and HCP marketing measurement. Key Takeaways: • **Veeva AI is the Next Platform Evolution:** Veeva is embedding Agentic AI deeply into the Vault platform (Model-Context-Protocol or MCP model), combining content, data, and agents. This platform-first approach ensures wide adoption across all 50+ Veeva applications and provides a "structural advantage" for building industry-specific solutions. • **Long-Term AI Value, Short-Term Focus on Execution:** While AI is projected to generate billions in industry value and significantly increase Veeva's market size, management does not expect material revenue contribution until FY 2027. IntuitionLabs.ai should focus on early adopter projects now to build expertise ahead of the broader market rollout. • **AI Targets Outsourced Labor in R&D:** Specific, aggressive goals include reducing outsourced labor and processing needed around the Clinical Trial Master File (TMF) by up to 50% through agent automation (e.g., document filing, quality checks, compliance tracking). This highlights a major automation opportunity for CROs and clinical operations. • **IQVIA Resolution Unlocks Commercial Suite:** The settlement allows full integration of industry-leading IQVIA data into Veeva Network and Veeva Nitro, removing a decade-long barrier. This revitalizes Veeva’s commercial analytics and data products, making the full commercial suite a more wholesome and competitive offering. • **Vault CRM Migration Momentum is Strong:** Veeva has secured commitments from nine Top 20 pharmaceutical companies for Vault CRM, significantly outpacing Salesforce (3 commitments). The successful go-live of two Top 20 customers in major markets validates the migration process and reduces perceived risk for remaining customers. • **Migration Timeline Confirmed:** The bulk of the remaining 300+ migrations from legacy Veeva CRM to Vault CRM are expected to occur in 2026 and 2027. This provides a clear window for IntuitionLabs.ai to ramp up its Veeva CRM consulting and migration services. • **AI Requires Business Consulting:** Every AI project is fundamentally a business consulting project because it necessitates redefining workflows and the division of labor between humans and agents. IntuitionLabs.ai should lead with consulting services focused on change management and process transformation to drive AI adoption. • **Crossix Drives Commercial Growth:** Crossix continues strong performance, driven by market share gains and an expanding product footprint, particularly in the high-growth Audiences (usage-based) and HCP marketing measurement segments. This indicates continued investment by pharma in digital marketing optimization. • **Quality Cloud Expansion:** Quality Cloud is receiving elevated focus, expanding into high-value areas like Laboratory Information Management Systems (LIMS) and batch release/validation management, presenting new opportunities for GxP and 21 CFR Part 11 compliance consulting. • **Agent Interoperability is Key:** Veeva is architecting its agents to communicate seamlessly with agents from other enterprise systems (e.g., SAP, Workday, Microsoft Co-Pilot) using a common protocol (MCP), making cross-system workflows much less brittle than traditional data integration methods. Tools/Resources Mentioned: * Veeva Vault Platform * Vault CRM * Veeva AI * Veeva Nitro (Commercial Analytics) * Veeva Network (Reference Data) * Crossix (Audiences, Measurement) * IQVIA Data * Veeva Quality Cloud (LIMS, Batch Release) * Veeva TMF (Trial Master File) Key Concepts: * **Industry Cloud Vision:** Veeva's strategy to combine industry-specific software, data, and business consulting into integrated suites for life sciences. * **Agentic AI:** Intelligent software agents that can act autonomously on behalf of a user within a workflow, interacting with content and data. * **Structural Advantage/Right to Win:** Veeva's competitive edge derived from its applications being the systems of record, allowing AI to be deeply embedded within the user's workflow, security, and transactional controls. * **MCP (Model-Context-Protocol):** A framework enabling easy agent-to-agent interoperability across different enterprise systems. Examples/Case Studies: * **Vault CRM Success:** Nine Top 20 pharmaceutical companies committed to Vault CRM; two Top 20 customers are now live in major markets. * **TMF Automation Goal:** Internal goal to reduce outsourced labor and processing related to Clinical TMF documentation by 50% using Agentic AI. * **IQVIA Partnership:** Joint calls between Veeva, IQVIA, and Top 20 pharma customers immediately following the resolution, signaling high customer interest in exploring new, integrated commercial solutions.

Veeva Systems VEEV Q2 2026 Earnings Call
Fyfull
/@fyfull
Aug 27, 2025
This video provides an in-depth exploration of Veeva Systems' fiscal 2026 second-quarter earnings call, offering significant insights into the company's strategic direction, product advancements, and market performance within the life sciences industry. The call, led by CEO Peter Gastner and other executives, highlights strong financial results and a clear vision for future growth, particularly emphasizing the transformative potential of AI, the strategic impact of resolving a long-standing dispute with IQVIA, and the continued momentum across its core product suites, including CRM, R&D, and Quality Cloud. A central theme of the discussion revolves around "Viva AI" and its integration into the Vault platform. Gastner articulates a vision where AI agents, content, and data will seamlessly interact, creating deep industry-specific applications and significantly expanding Veeva's market opportunity. While acknowledging that material revenue contribution from AI is not expected in the immediate future (2026-2027), the company anticipates creating billions of dollars in value for the industry by enhancing efficiency, automating tasks, and potentially reducing the need for certain types of outsourced labor in areas like safety and clinical operations. The discussion also touches upon the unique "structural advantage" Veeva possesses in AI due to its deeply embedded applications and system-of-record status within customer workflows. Another pivotal development discussed is the resolution of a decade-long lawsuit with IQVIA. This settlement is presented as a major unlock for Veeva's commercial cloud, removing previous restrictions that hindered the full potential of products like Veeva Network and Veeva Nitro, which can now integrate industry-leading IQVIA data. This move is expected to transform Veeva's commercial offerings, making them more comprehensive and practical for customers, fostering greater adoption of the entire commercial suite, and driving stickiness through enhanced business consulting. The call also provides updates on the competitive landscape for Veeva CRM, with the company reporting significant wins against Salesforce and successful go-lives with major pharmaceutical clients, underscoring the growing confidence in Vault CRM. Key Takeaways: * **Transformative AI Vision:** Veeva is making rapid progress on "Viva AI," deeply plumbing it into the Vault platform to create industry-specific agents that interact with content and data. This is expected to be transformative for the industry, generating billions in value by enhancing efficiency and automating tasks. * **Strategic IQVIA Resolution:** The settlement of the 10-year dispute with IQVIA is a major unlock for Veeva's commercial cloud. It removes artificial barriers, allowing products like Veeva Network and Veeva Nitro to integrate IQVIA data, making them more practical and comprehensive. * **Enhanced Commercial Cloud Potential:** The IQVIA resolution and the move to Vault CRM (away from Salesforce reliance) clear the runway for Veeva to significantly expand its commercial revenue by maturing existing applications (Campaign Manager, Service Center, Patient CRM) and driving customer adoption. * **Veeva CRM Dominance:** Veeva continues to gain traction with Vault CRM, reporting nine top 20 pharma companies committed, compared to Salesforce's three. Two top 20 customers are already live in major markets, demonstrating successful implementation and a faster time-to-value compared to Salesforce's longer projected timelines. * **Agentic AI Monetization Strategy:** Monetization of Viva AI will come from selling the platform for customers to create custom agents and, more importantly, from Veeva's industry-specific agents. While not expecting material revenue in 2026-2027, the long-term market size expansion is significant. * **Structural Advantage in AI:** Veeva's deep application integration, where its AI agents are built within existing systems of record (e.g., CRM, TMF), provides a "structural advantage." This allows agents to operate seamlessly within user workflows, adhere to security rules, and perform transactional updates. * **Diverse AI Use Cases:** Agentic AI is expected to impact all areas of life sciences differently. In commercial, it will enable greater productivity; in safety and clinical, it could significantly reduce outsourced labor (e.g., 50% reduction in TMF processing). * **Interoperable Agents:** Veeva is architecting its agents to communicate with agents from other systems (e.g., SAP, Workday, Microsoft Office) using a common protocol (MCP model), which promises less brittle and more seamless cross-system automation. * **Elevated Quality Cloud Focus:** Quality Cloud is receiving increased internal focus, with plans to extend into Laboratory Information Management Systems (LIMS), batch release, and validation management, indicating a significant growth area for Veeva. * **Business Consulting for AI Adoption:** Every AI project is inherently a business consulting project, as it involves changing human-agent workflows. Veeva's business consulting group plays a crucial role in guiding customers through this transformation, which is seen as a major structural advantage for AI adoption. * **Crossix as a Growth Driver:** Crossix, Veeva's commercial data and analytics product, continues to show strong momentum, driven by both market share gains and product footprint expansion (e.g., in audiences and HCP marketing). * **Migration Momentum for Vault CRM:** While some customers are hesitant to migrate from legacy Veeva CRM, the "pull of AI" and the natural progression of time are expected to accelerate migrations, with the bulk anticipated in 2026 and 2027. * **Measured AI Approach with Large Goals:** Veeva is taking a deliberate, platform-first approach to AI, but with very aggressive goals, such as potentially reducing outsourced labor in TMF by 50% across the industry. Partners are expected to play a role in developing agents and interoperating with Veeva's solutions. **Key Concepts:** * **Veeva AI / Agentic AI:** Veeva's proprietary artificial intelligence initiatives focused on creating industry-specific intelligent agents that automate tasks and enhance productivity within life sciences workflows. * **Vault Platform:** Veeva's foundational cloud platform that supports its suite of applications, integrating content, data, and now AI agents. * **Vault CRM:** Veeva's next-generation customer relationship management platform built on the Vault platform, designed specifically for the pharmaceutical industry. * **Veeva Network & Veeva Nitro:** Commercial data and analytics products that, following the IQVIA resolution, can now integrate industry-leading IQVIA data, enhancing their utility and effectiveness. * **Quality Cloud:** Veeva's suite of applications for managing quality processes, with an elevated focus on expanding into areas like LIMS (Laboratory Information Management Systems) and manufacturing. * **Crossix:** Veeva's commercial data and analytics business, focusing on measuring and optimizing marketing to both consumers (patients) and healthcare providers (HCPs). * **TMF (Trial Master File):** A critical collection of documents for clinical trials, an area identified for significant AI-driven automation. * **MCP Model (Context Protocol):** A common protocol enabling seamless agent-to-agent interoperability across different systems and vendors. * **Structural Advantage:** Veeva's inherent competitive edge in AI adoption due to its deep integration into customer workflows and its applications serving as systems of record. **Examples/Case Studies:** * **Vault CRM Go-Lives:** Two top 20 pharmaceutical companies successfully went live with Vault CRM in major markets within two years of commitment. * **TMF Processing Automation:** Agentic AI is envisioned to automate tasks such as document filing, verifying documentation completeness against protocols, and identifying illegible documents within the clinical trial master file. * **Commercial Content Workflow:** An early adopter AI project in commercial content involved intricate workflows between medical, regulatory, legal, different brands, and countries, demonstrating how AI and business consulting can transform complex processes. * **IQVIA Data Integration:** The resolution allows Veeva Network and Nitro to integrate IQVIA data, which was previously a "hole in our boat," making these products more viable and powerful for commercial analytics.
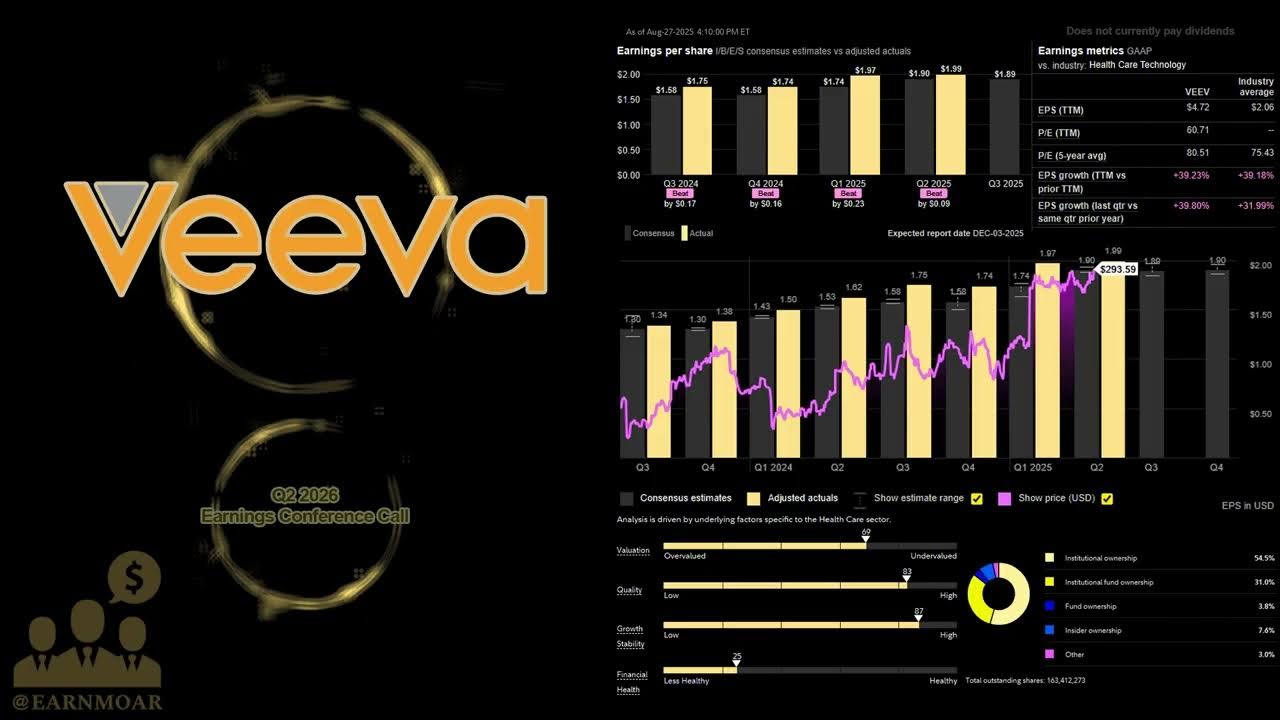
$VEEV Veeva Systems Q2 2026 Earnings Conference Call
EARNMOAR
/@EarnMoar
Aug 27, 2025
This video presents the Veeva Systems fiscal 2026 second quarter earnings conference call, offering a comprehensive update on the company's strategic direction, product advancements, and market performance within the life sciences industry. Key discussions revolved around the transformative potential of Veeva AI, the significant implications of resolving a long-standing lawsuit with IQVIA, and the continued momentum of Vault CRM and other cloud offerings. Management emphasized a vision where industry-specific software, data, and business consulting converge, with AI agents playing a pivotal role in enhancing efficiency and effectiveness across commercial, R&D, and quality operations, all while maintaining a focus on regulatory compliance. Key Takeaways: * **Transformative AI Strategy:** Veeva is making rapid progress on "Veeva AI," deeply embedding agentic AI into its Vault platform to create industry-specific agents. This is expected to be transformative for both Veeva and the life sciences industry, offering billions of dollars in value through increased human efficiency and automation of tasks, though material revenue contribution is not expected until 2027 or later. * **IQVIA Lawsuit Resolution Unlocks Commercial Cloud:** The resolution of the 10-year dispute with IQVIA removes "artificial barriers" that previously hindered Veeva's commercial cloud. This allows for the integration of industry-leading IQVIA data into Veeva Networks and Nitro, making these commercial analytics offerings practical and enabling a more "wholesome" solution that is expected to drive broader adoption of Veeva's commercial suite. * **Strong Vault CRM Momentum:** Veeva reports significant competitive success in the CRM market, with 9 top 20 pharma companies committed to Vault CRM, compared to Salesforce's 3. Two top 20 customers are already live in major markets, demonstrating proven implementation capabilities and a faster time-to-value compared to Salesforce's longer project timelines. * **Agentic AI Interoperability:** Veeva is architecting its AI agents to enable seamless communication and interoperability between agents within the Veeva ecosystem and with agents from other enterprise systems like SAP, Workday, or Microsoft. This "agent-to-agent interoperability" is highlighted as a less brittle and highly beneficial approach for cross-system workflows. * **Business Consulting Critical for AI Adoption:** The company stresses that every AI project is inherently a business consulting project, as it involves redefining workflows and the division of labor between humans and AI agents. This positions Veeva's business consulting services as a crucial component for successful AI implementation and change management within customer organizations. * **Broad-Based Growth Across Clouds:** Beyond commercial, Veeva sees continued strong execution and pipeline build in R&D subscriptions. Quality Cloud is also receiving elevated focus, with expansion into areas like Laboratory Information Management Systems (LIMS) and batch release, indicating its potential as a significant growth driver. * **Crossix Driving Commercial Growth:** Veeva's Crossix product continues to be a meaningful driver of commercial growth, showing strong performance in both its measurement business and the higher-growth audiences segment. This growth is attributed to increasing market share and expanding product footprint, particularly in HCP marketing.

Veeva (VEEV|$46.6B) - 2026 Q2 Earnings Analysis
SmartStockWatch
/@SmartStockWatch
Aug 27, 2025
This video provides an in-depth analysis of Veeva's fiscal 2026 second-quarter earnings report, presented by Smart Stockwatch with insights from senior analyst John. The primary purpose of the analysis is to dissect Veeva's financial performance, strategic initiatives, and future outlook, positioning it as a foundational leader in cloud solutions for the life sciences sector. The discussion covers key financial metrics, including revenue growth, profitability, and earnings per share (EPS), alongside significant product developments and partnerships that underscore Veeva's robust market position and growth trajectory. The analysis begins by highlighting Veeva's impressive financial results for Q2 2026, reporting total revenues of $789.1 million, a 17% year-over-year increase, largely driven by its subscription services which also grew by 17% to $659.2 million. Profitability metrics were equally strong, with non-GAAP operating income rising 26% to $352.6 million and non-GAAP net income increasing 25% to $333.4 million, reflecting effective cost management and operational efficiencies. A significant point of discussion was Veeva's adjusted EPS of $1.99, which substantially surpassed the analyst forecast of $1.34 by 65%, demonstrating the company's ability to exceed market expectations through strategic execution and a customer-centric approach. Beyond the financial figures, the video delves into Veeva's strategic initiatives and product developments. A key upcoming development is the launch of AI agents in December, designed to enhance their CRM and commercial content offerings, signaling a strong focus on leveraging artificial intelligence. The success of Vault CRM is also emphasized, now live with over 100 customers, solidifying its adoption in the industry. Furthermore, the resolution of legal disputes and subsequent partnership with IQVIA is presented as a major win, enabling seamless product integration for their joint customers. Veeva's role as a foundational player in drug development and quality is reinforced by the widespread adoption of its ETMF solutions by all top 20 biopharmaceutical companies, underscoring its critical contribution to modernizing clinical operations. The analysis concludes with an optimistic financial outlook, with Q3 revenue guidance set between $790-$793 million and full fiscal year 2026 revenues projected between $3.134 billion and $3.140 billion, driven by continuous innovation, particularly in AI, and an expanding CRM suite. Key Takeaways: * **Strong Financial Performance:** Veeva reported robust Q2 2026 results with $789.1 million in revenue (up 17% YoY) and a significant adjusted EPS of $1.99, far exceeding the analyst forecast of $1.34. This indicates strong operational efficiency and market demand. * **Subscription Services as Growth Driver:** The consistent 17% year-over-year growth in subscription services, reaching $659.2 million, highlights the stability and increasing demand for Veeva's cloud solutions within the life sciences industry. * **Strategic AI Integration:** Veeva is poised to launch AI agents in December, specifically designed to enhance its CRM and commercial content offerings, demonstrating a forward-looking strategy to leverage artificial intelligence for operational improvements. * **Widespread Vault CRM Adoption:** Vault CRM has achieved a significant milestone, being live with over 100 customers, which underscores its growing acceptance and utility as a leading platform in the pharmaceutical sector. * **Impactful IQVIA Partnership:** The resolution of previous legal disputes and subsequent partnership with IQVIA is a strategic victory, promising seamless product integration that will greatly benefit their shared customer base in the life sciences. * **Foundational Role in Drug Development:** Veeva's ETMF (Electronic Trial Master File) solutions have been adopted by all top 20 biopharmaceutical companies, solidifying its critical role in modernizing clinical operations, drug development, and quality management across the industry. * **Optimistic Future Outlook:** Veeva provided strong guidance for Q3 2026, expecting revenues between $790-$793 million and an adjusted EPS of $1.94-$1.95, reflecting confidence in sustained growth driven by innovation and market leadership. * **Innovation-Driven Growth:** The company's continued focus on innovation, particularly with AI and the expansion of its CRM suite, is identified as a key factor bolstering its growth prospects and market leadership in the life sciences cloud space. * **Customer-Centric Approach:** Veeva's ability to consistently deliver beyond market expectations and its strong customer base are attributed to its strategic execution and a deep focus on customer success. * **Market Demand for Cloud Solutions:** The overall strong performance and growth figures reflect a robust and increasing market demand for specialized cloud solutions in the life sciences industry, which Veeva is effectively capitalizing on. Key Concepts: * **Veeva (VEEV):** A leader in industry cloud solutions for the global life sciences industry, providing software and data solutions for pharmaceutical, biotech, and medical device companies. * **Veeva CRM:** A cloud-based customer relationship management solution specifically designed for the pharmaceutical and life sciences industries, helping sales teams manage customer interactions and content. * **Veeva Vault CRM:** An evolution of Veeva's CRM offerings, integrated with the broader Veeva Vault platform, providing enhanced capabilities for commercial content and customer engagement. * **AI Agents:** Artificial intelligence-powered tools designed to automate and enhance specific tasks, in this context, aimed at improving CRM and commercial content offerings. * **ETMF (Electronic Trial Master File) Solutions:** Digital systems used in clinical research to manage and store essential documents related to a clinical trial, ensuring compliance and efficiency in drug development. * **Life Sciences Cloud:** Cloud computing platforms and services tailored to meet the specific needs and regulatory requirements of the pharmaceutical, biotechnology, and medical device industries. Examples/Case Studies: * **Vault CRM Adoption:** Over 100 customers are currently live with Veeva's Vault CRM, demonstrating significant market penetration and successful implementation. * **ETMF Solutions in Top Biopharmas:** All top 20 biopharmaceutical companies have selected Veeva's ETMF solutions, highlighting the critical and foundational role Veeva plays in clinical operations and drug development for major industry players. * **IQVIA Partnership:** The successful resolution of legal disputes and subsequent partnership with IQVIA allows for seamless integration of their respective products, benefiting joint customers by providing a more unified solution ecosystem.

VEEV Stock HUGE News! (Buy Now or Wait?) Veeva Systems
mr. sicko Trading
/@mrsickotrading
Aug 27, 2025
This video provides a rapid technical analysis and trading prediction for Veeva Systems stock ($VEEV). The speaker, an experienced trader, outlines a specific, rules-based strategy for initiating a long position on the stock. The core objective is to capitalize on anticipated upward movement in Veeva’s valuation, but only under highly controlled entry conditions defined by technical indicators. The analysis is presented as a definitive, actionable trade plan rather than a general market commentary, emphasizing the speaker's personal methodology developed over thousands of prior analyses. The methodology hinges on identifying and respecting specific price action zones. The speaker indicates a clear bullish bias, stating, "Viva Systems, I'm looking to take it up." However, this bullish outlook is strictly conditional. The first step involves marking critical technical levels, specifically identifying a recent "top" and a key "demand" zone. This demand zone represents the price level where significant buying interest is expected to materialize. The speaker stresses that simply buying the stock randomly or chasing upward momentum is not a viable strategy; entry must be disciplined and systematic. The most crucial element of the strategy is the requirement for confirmation before execution. The speaker is waiting for the stock price to pull back to the previously identified demand zone. Once the price reaches this "stone" level, the trader is "activated" but still requires confirmation signals on smaller timeframes, specifically the 15-minute or 5-minute charts. This multi-timeframe approach is designed to minimize risk by ensuring that short-term momentum aligns with the larger structural support identified by the demand zone. The speaker explicitly warns against the common trading pitfall of "forcing trades" or "running after" a stock that gaps up, reinforcing the necessity of patience and adherence to predefined entry rules. Key Takeaways: • **Conditional Bullish Stance on $VEEV:** The analysis establishes a clear intent to take a long position on Veeva Systems stock, indicating a fundamental belief in the company's upward valuation potential, likely driven by its dominant position in the regulated life sciences software market. • **Importance of Technical Demand Zones:** The trading strategy relies heavily on identifying and marking a specific "demand" zone—a critical price level where institutional or significant buying pressure is expected to halt a pullback and reverse the trend. • **Multi-Timeframe Confirmation Requirement:** Entry into the trade is contingent upon receiving confirmation signals on lower timeframes (specifically 15-minute or 5-minute charts) after the price has reached the larger structural demand zone. This is a crucial risk mitigation step to ensure the larger trend is resuming. • **Disciplined Entry Strategy:** The speaker strongly advocates against impulsive trading, explicitly stating that "randomly buying my money is simply not going to happen." Success is tied to waiting for the market to meet the trader's predefined conditions, not chasing momentum. • **Avoiding Forced Trades (FOMO):** A key warning is issued against "running after" a stock that gaps up or moves without pulling back to the desired entry level. This prevents emotional trading driven by the Fear of Missing Out (FOMO) and ensures trades are based on technical merit. • **Rules-Based Execution:** The entire approach is centered on waiting for "rules" to be met. This highlights the necessity of having a formalized trading plan where entry, exit, and risk parameters are established before market engagement. • **Identifying the "Stone" Level:** The demand zone is referred to as the "stone"—the critical activation point where the trader shifts from observation to active waiting for confirmation, emphasizing the significance of this structural support level. • **Strategic Relevance of Veeva Systems:** Although the video is financial, the focus on $VEEV underscores the market's perception of the company, which provides the foundational CRM and compliance platforms that IntuitionLabs.ai specializes in consulting and integrating. Understanding the financial trajectory of this key partner is vital for long-term business strategy. Key Concepts: * **Demand Zone:** A technical analysis concept referring to a price area where buyers are concentrated, often leading to a reversal of a downward price movement due to strong buying interest. * **Confirmation:** The requirement for additional technical signals (e.g., candlestick patterns, volume spikes, or indicator crosses) on a smaller timeframe to validate the entry signal generated by the larger timeframe analysis. * **Timeframes (15-minute/5-minute charts):** Shorter-term charts used to pinpoint precise entry and exit points, reducing risk compared to entering based solely on daily or weekly charts. * **Technical Analysis:** The methodology of forecasting the direction of prices through the study of historical market data, primarily price and volume.

Lessons In Healthcare And Life, with Todd Whitthorne
Self-Funded
@SelfFunded
Aug 26, 2025
This video features a discussion on the intersection of personal well-being, effective leadership, and the economic realities of the U.S. healthcare system, emphasizing the critical role of employers in fostering a "culture of health." Guest Todd Whitthorne, drawing on his extensive background in prevention and executive roles at organizations like the Cooper Clinic and AAP Health, explores the systemic issues driving poor public health outcomes and escalating healthcare costs. The conversation establishes that a leader's personal health is a foundational asset, arguing that investing in self-care (fitness, nutrition, sleep, stress management, prevention) is not selfish but essential for professional success and modeling behavior. A significant portion of the discussion focuses on the complexities and inefficiencies of employer-sponsored healthcare. The speakers highlight the pervasive "point solution fatigue" among employers and consultants, where numerous niche solutions promise ROI but often fail due to low employee adoption and utilization. The crux of a successful solution, they argue, is effectively communicating its value and making it the "front door to all things healthcare," simplifying a complex system for the average employee. They introduce the concept of validating value propositions through objective financial ROI analysis, citing the work of the company Accorded, which uses actuarial science and claims data to independently verify if health solutions truly save money and measurably improve clinical outcomes. The conversation delves into two major public health crises directly impacting the workforce: the explosion of obesity and the rise of mental health challenges, particularly among younger generations (Gen Z). The speakers examine the economic and clinical dynamic of GLP-1 weight loss drugs, noting the striking cost implications for employer health plans (citing a state plan facing a \$250-\$300 million expense). They stress that while GLP-1s are efficacious, they must be bundled with behavioral change, coaching, and nutrition to ensure sustained weight loss, as long-term data on their effects is still being earned. Furthermore, the discussion touches on the mental health crisis, linking it to the "death of the play-based childhood" and the pervasive influence of social media, which has led to increased isolation, anxiety, and depression in the workforce, creating a massive supply-and-demand imbalance for qualified clinicians. The speakers conclude by advocating for a shift in leadership focus toward three key buckets of ROI: Financial ROI, Clinical ROI (measurable health improvement), and Cultural ROI. Cultural ROI involves demonstrating genuine care for employees' health and well-being, which builds trust and translates into tangible business benefits like improved recruitment, retention, engagement, and productivity. This intentional investment in people is cited as a consistent predictor of a more profitable company, reinforcing the idea that organizational success is inextricably linked to the health and vitality of its workforce. Key Takeaways: * **The Three Buckets of ROI:** Leaders should evaluate health solutions based on three returns: Financial ROI (cost savings), Clinical ROI (measurable health improvement), and Cultural ROI (building trust and signaling care to the workforce). * **Combatting Point Solution Fatigue:** The success of any employer-sponsored health solution hinges on high adoption and utilization. Solutions must cut through the clutter, effectively communicate their value, and strive to become the singular "front door" for all employee healthcare interactions. * **The Business Case for Health Investment:** Studies consistently show that a healthier company is a more profitable company, demonstrating measurable and significant improvements in productivity, engagement, recruitment, retention, and ultimately, sales and EBITDA. * **Insist on Validated ROI:** Employers should push back on vendors and demand that new solutions be piloted at the vendor's expense with performance guarantees. Independent, objective validation (using claims data and actuarial math) is necessary to confirm if a point solution truly delivers financial and clinical value. * **GLP-1s and the Economic Challenge:** The rapid adoption and high cost of GLP-1 drugs (e.g., semaglutides) pose a massive economic challenge to employer health plans, potentially adding hundreds of millions of dollars to annual spend. This cost cannot be ignored, requiring careful consideration of coverage and payment strategies. * **GLP-1s Require Bundled Care:** GLP-1s should be viewed as a catalyst for weight loss, not a standalone solution. They must be tied into comprehensive behavioral change programs, including coaching, nutrition, and exercise, to maximize effectiveness and sustain weight loss, especially since they are designed to be taken for life. * **The Crisis of Mental Health in the Workforce:** The rise of anxiety and depression, particularly among younger employees (Gen Z, those born after 1995), is a critical workplace issue. Leaders must be mindful of and sensitive to the unique struggles of this demographic, recognizing the need for intentional mental health support. * **Fitness Trumps All Predictors of Longevity:** Objectively measured fitness (like cardiovascular capacity) is the single greatest predictor of how long and how well an individual will live, influencing "healthspan" (quality years) over mere "lifespan." * **Personal Investment is Not Selfish:** Leaders must prioritize their own fitness, nutrition, sleep, and stress management. This self-investment is necessary to show up with the energy and resilience required to lead and positively influence others. * **The Power of Eudemonic Happiness:** True, deep well-being (eudemonic happiness) stems from a life of meaning and purpose, which is ultimately rooted in the richness of human relationships and connection, not hedonistic pursuits or material success. * **Prioritize HR Resources:** HR departments bear a substantial burden managing complex healthcare systems. Leaders should view HR as a critical, non-revenue-generating investment and provide them with the necessary resources and weapons to effectively manage benefits and improve employee lives. Tools/Resources Mentioned: * **Accorded:** A company that uses actuarial science to independently validate the financial and clinical ROI of point solutions for employers. * **Healthy/VEXA:** Mentioned as examples of health solutions. * **Claim Doc, Nomi Health, Paro Health:** Companies involved in medical claim auditing, self-funded benefits, and benefits captives. * **The Daily Stoic / Daily Dad:** Resources based on Stoic philosophy. * **The Anxious Generation (by Jonathan Height):** Book documenting the mental health struggles of those born after 1995. * **Harvard Adult Development Study:** Longitudinal study on the determinants of human happiness (concluding that relationships are key). Key Concepts: * **Healthspan vs. Lifespan:** The difference between the total years lived (lifespan) and the number of years lived in good health and without restriction (healthspan). * **Point Solution Fatigue:** The exhaustion and frustration experienced by employers and consultants due to the overwhelming number of niche health and wellness vendors offering specialized solutions. * **Cultural ROI:** The return on investment derived from demonstrating genuine care for employees, leading to improved trust, engagement, and organizational culture. * **Eudemonic Happiness:** A state of deep well-being derived from living a life of meaning, purpose, and virtue, often contrasted with immediate, pleasure-seeking (hedonistic) happiness. * **Metabolic Syndrome:** A cluster of five risk factors (triglycerides, blood pressure, glucose, waist circumference, HDL cholesterol) that are strong predictors of future large healthcare claims and chronic disease development. Examples/Case Studies: * **70-Year-Old Cyclist Resilience:** A real-world story of a 70-year-old man who, due to his lifelong commitment to fitness, survived being hit and run over by a drunk driver while cycling and was wake surfing two weeks later, illustrating the resilience conferred by physical health. * **Purdue University Health Plan:** Cited as an example of an organization that successfully kept healthcare costs low by heavily investing in its internal benefits resources (18 full-time benefits staff for 18,000 employees). * **GLP-1 Cost Example:** A state health plan was calculated to face a \$250-\$300 million annual expense due to approximately one-third of its population utilizing GLP-1 weight loss medications.

Leadership Shift: From Control to Coordination 🚀
Drug Diaries
/@DrugDiaries
Aug 19, 2025
This video provides an in-depth exploration of the evolving landscape within the pharmaceutical industry, emphasizing a critical leadership shift from a control-oriented, linear process to a more agile, coordinated approach. Florian Schnappauf discusses the significant challenges and opportunities facing pharma companies as they brace for an unprecedented influx of product launches by 2030, highlighting the imperative for operational transformation without commensurate increases in resources. The discussion establishes that success in this new era hinges on agility in decision-making, rapid reallocation of priorities, and fostering interconnected functions across the organization. A central theme of the conversation revolves around optimizing commercial operations and enhancing customer engagement, particularly with healthcare professionals (HCPs). Schnappauf details the complexities introduced by specialty medicines, which demand more nuanced and integrated strategies than traditional launch playbooks. He underscores the necessity of creating a unified view of HCPs, leveraging technology to personalize interactions and improve the overall HCP experience. Furthermore, the video delves into the pivotal role of effective content management in driving engagement and the transformative impact of artificial intelligence (AI) on pharma interactions, offering practical AI applications within CRM systems. The discussion also provides significant insights into Veeva's strategic evolution, specifically its move to the Vault platform for CRM. This transition is presented as a critical development for the industry, with Schnappauf detailing the milestones in Vault CRM adoption, customer feedback, and the migration challenges companies face. He elaborates on how AI is being integrated into CRM to enhance capabilities, including voice control for compliance and innovations in campaign management and service centers. Ultimately, the video culminates in a call for fundamental mindset shifts among pharma leaders to successfully navigate the complexities of the future and redesign launch strategies for sustained success by 2030. Key Takeaways: * **Mindset Shift from Control to Coordination:** Pharma leaders must transition from tightly managed, linear processes to agile, connected ways of working. This involves faster decision-making and quicker reallocation of priorities to adapt to market dynamics and resource constraints. * **The 2030 Launch Challenge:** The pharmaceutical industry is facing an unprecedented volume of new product launches by 2030, creating immense pressure for success without a proportional increase in resources. This necessitates innovative and efficient launch strategies. * **Complexity of Specialty Medicines:** Specialty medicines present unique challenges that traditional launch playbooks are ill-equipped to handle. Their specific patient populations, complex administration, and high-touch support requirements demand tailored, integrated approaches. * **Risks of Traditional Launch Playbooks:** Relying on outdated, linear launch strategies in a rapidly evolving market can lead to inefficiencies, missed opportunities, and ultimately, unsuccessful product introductions. Agility and adaptability are crucial. * **Connecting Functions for Smarter Launches:** Breaking down silos and fostering interconnectedness across various organizational functions (e.g., commercial, medical, regulatory) is essential for developing and executing smarter, more integrated launch strategies. * **Creating a Unified View of HCPs:** It is critical to integrate data from disparate sources to build a comprehensive, unified profile of healthcare professionals. This holistic view enables more personalized, relevant, and impactful engagements. * **Enhancing HCP Experience Through Technology:** Leveraging technology is key to improving interactions with HCPs, providing them with valuable, timely information, and streamlining communication channels to foster stronger relationships. * **The Pivotal Role of Content in Pharma Engagement:** Effective content management is vital for engaging HCPs and patients. Content must be relevant, accessible, compliant, and delivered through appropriate channels to drive meaningful interactions. * **The Impact of AI on Pharma Interactions:** Artificial intelligence is transforming how pharma companies interact with customers, enabling enhanced customer engagement through personalized communication, predictive analytics, and automated support. * **Veeva's Strategic Move to Vault Platform:** Veeva's shift to its own Vault platform for CRM is a significant industry development. Companies must understand the implications for adoption, migration challenges, and the opportunities this platform offers for integrated operations. * **Practical AI Applications in CRM:** AI can be practically applied within CRM systems to automate tasks, provide intelligent insights for sales and marketing, personalize customer journeys, and improve overall operational efficiency. * **Voice Control and Compliance Innovations:** AI-powered voice control offers new avenues for efficiency and compliance in pharma interactions, enabling easier data capture and ensuring adherence to stringent regulatory requirements. * **Innovations in Campaign Manager and Service Center:** AI and advanced technologies are driving innovations in campaign management, allowing for more targeted and effective marketing efforts, and in service centers, enhancing support for HCPs and patients. * **Redesigning Pharma Launch Strategies:** The future demands a fundamental redesign of pharma launch strategies, moving away from rigid models towards more flexible, data-driven, and customer-centric approaches that can adapt quickly to market feedback. Tools/Resources Mentioned: * Veeva CRM * Veeva Vault platform * Artificial Intelligence (AI) * Voice Control technology Key Concepts: * **Control to Coordination Shift:** A strategic organizational shift from hierarchical, top-down management to a more collaborative, agile, and interconnected operational model. * **Agile Operations:** The ability of an organization to respond quickly and effectively to changes in the market, technology, or customer needs through flexible processes and rapid decision-making. * **Unified HCP View:** An integrated, comprehensive profile of healthcare professionals, compiled from various data sources, to enable a holistic understanding and personalized engagement strategy. * **Specialty Medicines:** Pharmaceutical products designed to treat complex, chronic, or rare conditions, often requiring specialized administration, patient support programs, and unique commercial strategies.

Veeva’s 10-Year Mission: Building the Industry Cloud for Life Sciences 🌐
Drug Diaries
/@DrugDiaries
Aug 19, 2025
This video provides an in-depth exploration of Veeva’s strategic vision—the "Industry Cloud for Life Sciences"—and the necessary operational and cultural transformation required for pharmaceutical companies to succeed in the coming decade. The discussion, featuring Florian Schnappauf, frames the current moment as a critical crossroad, emphasizing that traditional commercial models are insufficient to handle the anticipated influx of product launches by 2030. The core message centers on the need for pharmaceutical leaders to adopt a new mindset focused on connected functions, data unification, and the strategic integration of artificial intelligence to enhance customer engagement and operational efficiency. A major theme is the "2030 Launch Challenge," driven by the increasing complexity of specialty medicines. These products require highly targeted, sophisticated engagement strategies that traditional, siloed launch playbooks cannot support. The video stresses that mitigating the risks of these traditional approaches requires connecting disparate functions—such as commercial, medical affairs, and regulatory—to ensure smarter, compliant, and coordinated market entry. This operational cohesion is essential for managing the complexity inherent in specialty medicine distribution and patient support. To facilitate this transformation, the discussion highlights the critical role of technology in creating a unified view of Healthcare Professionals (HCPs). Achieving a seamless, personalized HCP experience depends on robust data engineering and effective content management. Furthermore, the video details Veeva's significant strategic move to migrate its CRM capabilities onto the native Vault platform. This shift is designed to unify data, content, and processes within the broader Industry Cloud vision, thereby providing a single source of truth for commercial operations and streamlining compliance. Crucially, the video explores the transformative impact of AI on pharma interactions and commercial operations. It delves into the practical applications of AI within CRM, such as automating routine tasks, providing intelligent sales assistance, and streamlining compliance tracking. Innovations like voice control and enhanced campaign management tools are presented as key components of the next generation of pharma technology, enabling field teams to operate more efficiently while maintaining strict adherence to regulatory standards. Ultimately, the success of pharma companies by 2030 hinges on their willingness to embrace these technological and mindset shifts. Key Takeaways: • **Veeva’s Industry Cloud Vision:** Veeva’s long-term North Star is to build the comprehensive industry cloud for life sciences, unifying data, content, and processes across commercial, clinical, and regulatory functions to create a seamless operational environment. • **The 2030 Launch Imperative:** The pharmaceutical industry faces an impending wave of product launches, primarily specialty medicines, necessitating a fundamental redesign of launch strategies to move beyond traditional, siloed playbooks which are now considered high-risk. • **Mindset Transformation is Key:** Success by 2030 requires pharma leaders to shift their organizational mindset, prioritizing connected functions, integrated data, and a customer-centric approach over legacy operational structures. • **Strategic Shift to Vault CRM:** Veeva is strategically migrating its CRM capabilities onto the native Vault platform to achieve greater unification of data and content, which is essential for maximizing CRM investment and ensuring compliance within the Industry Cloud framework. • **Unified HCP View is Essential:** Creating a single, unified view of Healthcare Professionals (HCPs) is critical for enhancing engagement quality, personalization, and experience, requiring robust data integration and business intelligence capabilities. • **AI’s Role in Commercial Operations:** AI is poised to significantly impact pharma interactions by enabling intelligent automation, enhancing customer engagement, and providing practical assistance within the CRM system, such as generating sales insights and automating compliance checks. • **Content Management Centralization:** Effective content management is highlighted as a foundational element for improving HCP engagement, ensuring that the right, compliant information reaches the right professional at the right time. • **Connecting Functions for Compliance:** Smarter launches and operations require connecting traditionally separate functions (e.g., commercial and medical affairs) to ensure that all activities are coordinated and compliant, particularly important for complex specialty medicines. • **Practical AI Applications in CRM:** Specific applications of AI include streamlining field force operations, automating data entry through voice control, and enhancing audit trails and compliance tracking to meet GxP and 21 CFR Part 11 requirements. • **Innovations in Commercial Tools:** New components like Campaign Manager and Service Center are crucial parts of the broader Industry Cloud vision, designed to provide advanced tools for marketing orchestration and customer service within the regulated environment. Tools/Resources Mentioned: * **Veeva Vault Platform:** The foundational cloud platform for Veeva’s Industry Cloud strategy, now hosting CRM capabilities. * **Vault CRM:** The next generation of Veeva’s customer relationship management solution, built natively on the Vault platform. * **Campaign Manager:** A specific innovation designed to enhance marketing orchestration and execution within the Veeva ecosystem. * **Service Center:** A tool mentioned as part of the broader vision to improve customer service and support functions for life sciences companies. Key Concepts: * **Industry Cloud for Life Sciences:** Veeva’s overarching vision to create a unified, comprehensive cloud environment that connects all critical functions (R&D, Clinical, Commercial, Regulatory) for pharmaceutical and biotech companies. * **2030 Launch Challenge:** The anticipated operational and strategic difficulties pharmaceutical companies will face due to the high volume and complexity of product launches expected by the end of the decade, particularly in specialty medicines. * **Unified View of HCPs:** The goal of integrating data from all touchpoints (CRM, medical affairs, digital channels) to create a single, comprehensive profile of each Healthcare Professional to enable personalized and relevant interactions. * **Connected Functions:** The operational shift required to break down organizational silos, ensuring that commercial, medical, and regulatory teams work together seamlessly to support product launches and ongoing operations.

How Veeva AI Is Powering 50+ Apps on a Single Platform 🤖
Drug Diaries
/@DrugDiaries
Aug 19, 2025
This video provides an in-depth exploration of the strategic transformation underway in the pharmaceutical industry, driven by the anticipated influx of product launches by 2030 and Veeva’s foundational shift to integrate AI capabilities across its unified Vault platform. The discussion, led by Florian Schnappauf, frames the current moment as a critical crossroad for life sciences companies, necessitating a fundamental change in mindset and a move away from traditional, siloed launch playbooks. The central theme is the necessity of connecting commercial, medical, and clinical functions to enable smarter, more efficient operations, with the Veeva Vault platform serving as the technological backbone for this integration. The speaker emphasizes that this shift is crucial for managing the complexity introduced by specialty medicines and ensuring effective, compliant customer engagement. A significant portion of the conversation details Veeva’s strategic move to consolidate all its offerings—including the historically separate CRM—onto the proprietary Vault platform. This initiative, powered by a dedicated organization and substantial budget under "Veeva AI," aims to bring platform-level AI capabilities to its entire portfolio of 50+ applications. By unifying the platform, Veeva can ensure that AI tools and data insights are seamlessly shared across applications, from content management and regulatory compliance to commercial operations. This integration is designed to create a unified, 360-degree view of healthcare professionals (HCPs), moving beyond simple transactional data to enhance the quality and relevance of every interaction, thereby improving the overall HCP experience. The practical application of AI within this new framework is highlighted through several examples focused on optimizing commercial operations and compliance. The discussion covers how AI can enhance customer engagement, streamline content creation and approval workflows, and introduce innovations like voice control for compliant field interactions. Specifically, AI’s role in CRM is explored, focusing on intelligent automation for sales operations, such as generating personalized sales content or automating routine tasks. Furthermore, the video addresses the challenges of migration to Vault CRM, noting the importance of customer feedback and managing the transition while maintaining operational continuity and adherence to strict regulatory standards (GxP, 21 CFR Part 11). Ultimately, the analysis concludes with a call for pharma leaders to adopt a new mindset focused on agility, connectivity, and leveraging technology to redesign launch strategies for the complex market of 2030. Key Takeaways: * **The 2030 Launch Challenge:** The pharmaceutical industry faces a significant operational challenge due to the predicted surge in product launches by 2030, demanding a complete overhaul of traditional, linear launch strategies in favor of connected, agile operations. * **Veeva’s Platform Unification Strategy:** Veeva is strategically migrating all 50+ applications, including its core CRM, onto the unified Vault platform, creating a single, regulated environment where data and processes are inherently connected across commercial, medical, and clinical functions. * **Veeva AI as a Platform Layer:** The dedicated Veeva AI organization is focused on embedding AI capabilities at the platform level, ensuring that all applications—from content management to CRM—can utilize shared AI services for enhanced automation, prediction, and intelligence. * **AI’s Role in Commercial Operations:** Practical AI applications in CRM include intelligent automation for sales operations, such as generating personalized content recommendations for field reps, optimizing call planning, and automating administrative tasks to increase selling time. * **Unified HCP View:** Achieving a single, unified view of Healthcare Professionals (HCPs) is critical for improving engagement; the integrated Vault platform facilitates this by breaking down data silos that traditionally separate commercial, medical, and content interaction data. * **Specialty Medicine Complexity:** The rise of specialty medicines presents a dual challenge, requiring more sophisticated and personalized engagement strategies that traditional launch playbooks are ill-equipped to handle, emphasizing the need for data-driven, connected functions. * **Content Management is Key to Compliance:** Effective content management is foundational to compliant customer engagement; AI can streamline the creation, review, and approval of promotional and medical content, ensuring rapid deployment while maintaining regulatory adherence. * **Innovation in Compliance and Field Use:** Innovations like voice control integration within the CRM are being developed to allow field teams to capture interaction details compliantly and efficiently, minimizing manual data entry and improving data quality. * **Mindset Shift for Pharma Leaders:** Success by 2030 requires pharma leaders to shift their mindset from siloed departmental thinking to one focused on cross-functional connectivity, embracing technology as a core strategic asset rather than just an IT tool. * **Vault CRM Migration Considerations:** While the move to Vault CRM offers significant long-term benefits, companies must carefully manage the migration process, addressing customer feedback and ensuring that the transition maintains GxP and 21 CFR Part 11 regulatory compliance. Tools/Resources Mentioned: * Veeva Vault Platform * Veeva CRM * Veeva AI * Campaign Manager (Veeva application) * Service Center (Veeva application) Key Concepts: * **Vault Platform:** Veeva’s proprietary cloud platform designed specifically for the life sciences industry, serving as the common technological foundation for all its applications, ensuring integrated data and compliance management. * **Veeva AI:** A dedicated initiative within Veeva focused on embedding advanced AI and machine learning capabilities directly into the Vault platform layer, making these intelligent services accessible across the entire suite of 50+ applications. * **Unified View of HCPs:** The strategic goal of integrating all data points (commercial interactions, medical inquiries, content consumption, clinical trial participation) related to a specific Healthcare Professional into a single, actionable profile to enable highly personalized and compliant engagement.

$20B in Pharma Content — But 75% Is Never Used 🤯
Drug Diaries
/@DrugDiaries
Aug 19, 2025
This video provides an in-depth exploration of the strategic crossroads facing the pharmaceutical industry, driven by the impending wave of product launches anticipated by 2030 and the urgent need for operational modernization. The speaker, Florian Schnappauf, establishes the context by highlighting a critical inefficiency within commercial operations: the industry invests approximately $20 billion annually in content production, yet a staggering three-quarters of this material is either never or almost never used. This massive content waste signals a fundamental disconnect between production volume and customer relevance, necessitating a profound shift in mindset and strategy across life sciences organizations. The discussion centers on the necessity of moving beyond traditional, siloed launch playbooks, which are proving inadequate for the complexity of specialty medicines and the evolving regulatory landscape. Schnappauf argues that successful future launches require connecting disparate functions—specifically commercial, medical affairs, and regulatory compliance—to ensure content is not only relevant but also compliant and delivered efficiently. A key technological enabler for this transformation is the creation of a unified, 360-degree view of the Healthcare Professional (HCP). This unified data foundation is essential for tailoring interactions, optimizing the customer experience, and maximizing the utility of the content that is produced. A significant portion of the conversation focuses on the strategic evolution of enterprise technology, particularly Veeva’s move to consolidate its offerings onto the Vault platform for CRM. This shift is presented not merely as a technical upgrade but as a strategic imperative designed to foster the necessary connectivity and compliance required for modern pharma operations. The video explores the milestones, customer feedback, and challenges associated with this migration, emphasizing how the Vault platform integrates content management (PromoMats), CRM, and clinical data to streamline processes. Furthermore, the discussion details the transformative impact of AI and LLMs on the CRM ecosystem, outlining practical applications such as generative AI tools for sales operations, voice control for compliant field reporting, and enhanced campaign management capabilities designed to increase content relevance and reduce the $20 billion content waste problem. Key Takeaways: • **The $20 Billion Content Crisis:** The pharmaceutical industry spends an estimated $20 billion annually on content production, but over 75% of this content is never utilized, highlighting a massive inefficiency in commercial operations and a failure to achieve content relevance with HCPs. • **The 2030 Launch Challenge:** Pharma companies must abandon traditional, siloed launch strategies to prepare for the anticipated influx of product launches by 2030, requiring integrated planning across commercial, medical, and regulatory functions. • **Mandate for Connected Functions:** Success in the modern life sciences environment hinges on connecting previously siloed functions (e.g., Medical Affairs and Commercial Operations) to ensure smarter, compliant, and more efficient product launches and ongoing engagement strategies. • **Unified HCP View is Essential:** Creating a comprehensive, unified view of the Healthcare Professional (HCP) is critical for enhancing the customer experience, enabling personalized interactions, and ensuring that sales and medical teams deliver relevant content at the right time. • **Veeva's Strategic Vault Migration:** Veeva’s move to its proprietary Vault platform for CRM is a strategic industry shift aimed at providing a more connected, compliant, and integrated ecosystem, particularly beneficial for managing content (PromoMats) and regulatory requirements. • **AI as the Relevance Engine:** Artificial Intelligence, particularly Generative AI and LLMs, is positioned as the key technology to move beyond mass content production to personalized engagement, automating tasks and providing intelligent assistance within the CRM environment. • **Practical AI Applications in CRM:** Specific applications of AI include creating Generative AI Sales Ops Assistants to streamline administrative tasks, intelligent automation for audit trails, and voice control features that ensure compliant data capture in the field. • **Compliance Innovation:** New technological features, such as voice control integrated into CRM, must be designed with regulatory compliance (e.g., 21 CFR Part 11, GxP) built-in to ensure accurate and auditable data capture during field interactions. • **Specialty Medicine Complexity:** The rise of specialty medicines introduces dual challenges related to complex scientific communication and highly targeted customer segments, demanding more sophisticated and relevant content strategies than traditional primary care drugs. • **Mindset Shift for Leaders:** Pharma leaders must adopt a transformative mindset that prioritizes connectivity, data integration, and technological innovation to redesign launch strategies and optimize commercial operations for the next decade. • **Leveraging Campaign Manager and Service Center:** Innovations in platforms like Veeva’s Campaign Manager and Service Center are crucial for improving the orchestration of customer journeys and ensuring that content delivery is targeted and measurable, directly addressing the content waste issue. Tools/Resources Mentioned: * Veeva Vault CRM * Veeva PromoMats (Implied, as part of content management) * Veeva Campaign Manager * Veeva Service Center * Large Language Models (LLMs) and Generative AI Key Concepts: * **Unified View of HCPs:** A comprehensive, integrated data profile of a Healthcare Professional that combines information from commercial, medical, and potentially clinical sources to provide a single source of truth for all engagement activities. * **2030 Launch Challenge:** The strategic and operational challenge facing the pharmaceutical industry due to the anticipated high volume and complexity of product launches in the coming years, requiring a shift from traditional, sequential processes to integrated, agile strategies. * **Content Relevance:** The measure of how useful, timely, and specific pharmaceutical content is to the target audience (HCPs), contrasting sharply with the current industry standard of high volume and low utilization.

Why Veeva Chose to Become a Public Benefit Corporation 💡
Drug Diaries
/@DrugDiaries
Aug 19, 2025
The video provides an expert analysis of the strategic transformation required within the pharmaceutical and life sciences industries, particularly in preparation for the anticipated surge of product launches expected by 2030. Featuring insights from Florian Schnappauf, the discussion establishes that the industry is at a critical crossroad, demanding a fundamental shift in traditional mindsets and operational strategies to manage this influx successfully. The core argument centers on the necessity of moving beyond siloed functions, leveraging advanced technology like AI, and understanding the strategic implications of Veeva’s platform evolution. A significant portion of the conversation addresses the operational challenges inherent in the current landscape, specifically highlighting the complexity introduced by specialty medicines and the high risks associated with relying on outdated launch playbooks. The solution proposed is the integration of commercial, medical, and regulatory functions to enable "smarter launches." This connectivity is crucial for creating a unified, comprehensive view of Healthcare Professionals (HCPs). The speaker emphasizes that technology must be deployed not just for data collection, but to genuinely enhance the HCP experience, moving toward personalized and valuable interactions, supported by effective and compliant content management. The video details Veeva’s strategic evolution, including its transition to operating as a Public Benefit Corporation (PBC). This corporate structure guides Veeva’s mission to prioritize the long-term benefit of the life sciences industry, its customers, and its employees, rather than solely focusing on shareholder value. This PBC status is presented as the philosophical underpinning that allows Veeva to innovate deeply and specifically for the regulated life sciences sector, justifying its strategic move to position the proprietary Vault platform as the future foundation for CRM. The discussion covers the milestones and challenges associated with customer migration to Vault CRM and concludes with a strong focus on the practical application of AI within the CRM environment—including voice control, compliance innovations, and intelligent automation—as essential components for future commercial success. Key Takeaways: • The pharmaceutical industry faces a significant "2030 Launch Challenge," requiring companies to abandon traditional, siloed launch playbooks in favor of integrated, cross-functional strategies to handle the expected volume of new products, especially specialty medicines. • Veeva's designation as a Public Benefit Corporation (PBC) signifies a commitment to industry-centric innovation, meaning their platform roadmap prioritizes solutions that benefit the life sciences ecosystem and regulatory compliance over short-term financial gains. • Effective commercial strategy requires connecting previously siloed functions—such as Medical Affairs, Commercial Operations, and Regulatory—to ensure seamless data flow and coordinated engagement throughout the product launch lifecycle. • Creating a unified view of Healthcare Professionals (HCPs) is paramount; technology must integrate data across all touchpoints to move beyond basic contact management and deliver a consistently enhanced, personalized HCP experience. • AI is rapidly transitioning from theoretical concept to practical application within CRM, enabling intelligent automation for sales operations, enhancing customer engagement, and providing field teams with actionable, real-time insights. • The strategic migration to Veeva Vault CRM is positioned as a necessity for future innovation, requiring pharmaceutical companies to plan for adoption milestones and proactively manage the associated customer feedback and migration challenges. • Content management is identified as a critical bottleneck; technology solutions must streamline the creation, regulatory approval, and distribution of compliant content to support personalized interactions and maximize engagement efficiency. • Specialty medicines present a dual challenge—clinical complexity combined with specialized commercialization needs—demanding bespoke technology solutions that standard, generic CRM platforms cannot adequately address. • Future CRM innovation, such as voice control integration and automated audit trails, is crucial for improving field force efficiency while simultaneously ensuring strict adherence to regulatory standards like GxP and 21 CFR Part 11. • Pharma leaders must undergo a fundamental mindset shift by 2030, embracing technology-driven redesigns of launch strategies to ensure operational agility and competitive advantage in a highly regulated and rapidly evolving market. Tools/Resources Mentioned: * Veeva Vault Platform * Veeva CRM (Vault CRM) * Campaign Manager (Veeva) * Service Center (Veeva) Key Concepts: * **Public Benefit Corporation (PBC):** A type of for-profit corporate entity that includes positive impact on society, workers, the community, and the environment in addition to profit as its legally defined goals. Veeva adopted this structure to guide its mission for the life sciences industry. * **Unified View of HCPs:** The concept of integrating all data points (commercial, medical, digital interactions) related to a Healthcare Professional into a single, comprehensive profile to enable coordinated and relevant engagement across all company functions. * **Connected Functions:** The operational strategy of breaking down traditional organizational silos (e.g., between commercial, medical, and regulatory teams) to ensure integrated planning and execution, particularly crucial for complex product launches.

Advanced Safety Database Configuration in Pharmacovigilance: Key Principles Explained
Pharmuni
/@pharmuni
Aug 18, 2025
This analysis provides an in-depth explanation of the key principles necessary for configuring an advanced, compliant safety database within the pharmacovigilance (PV) landscape. The core premise is that the safety database is the central engine driving regulatory compliance, supporting global reporting requirements, and ultimately safeguarding patients. The video establishes that a well-configured system ensures teams meet deadlines, minimize errors, and produce inspection-ready records, contrasting this with the risks of an inflexible or incomplete setup, which leads to delays, inconsistencies, and regulatory findings. The foundation of a compliant safety database configuration rests on robust regulatory alignment and process mapping. The system must be capable of capturing all required data for every regulatory region, specifically adhering to international standards such as E2BR3 for electronic case reporting. Beyond global standards, the configuration must be flexible enough to adapt to local requirements, including varying timelines, seriousness criteria, and specific submission formats. Crucially, the configuration must precisely mirror the company's internal processes, including Standard Operating Procedures (SOPs), affiliate workflows, and product-specific reporting logic. The speaker emphasizes that a rigid or poorly mapped configuration is a direct path to missed deadlines, duplicated effort, and adverse inspection outcomes. Operational efficiency and data integrity are secured through strategic automation and stringent control mechanisms. The system configuration should leverage automation to handle routine tasks such as case routing, report triggering, and supporting validation activities, thereby reducing reliance on error-prone manual steps. However, this automation must be underpinned by comprehensive audit trails, which are essential for regulatory accountability. Furthermore, access control must be granular, defined strictly by user roles to ensure that individuals only interact with necessary data and functions. The final principle highlights that a safety database is not a static asset but a "living system." Compliance requires continuous adaptation to evolving regulations, necessitating careful planning for updates, proactive risk identification, thorough validation of all changes, and meticulous documentation throughout the system lifecycle. Key Takeaways: * **Safety Database as Compliance Driver:** The safety database is the critical system that ensures regulatory accountability, supports global reporting obligations, and is foundational to patient safety protocols within pharmacovigilance. * **E2BR3 Adherence is Mandatory:** Configuration must be built to capture all necessary data fields to meet E2BR3 standards, which govern the electronic transmission of individual case safety reports (ICSRs) globally. * **Process Mapping is Non-Negotiable:** The database configuration must be meticulously mapped to internal company processes, including specific SOPs, affiliate workflows, and product-specific logic, to prevent operational friction and regulatory non-compliance. * **Risk of Rigidity:** A configuration that is too rigid or poorly aligned with real-world workflows will inevitably lead to operational inefficiencies, missed deadlines, duplicated work, and potential inspection findings. * **Automation for Efficiency and Compliance:** A well-configured system should automate case routing, report generation, and validation processes, minimizing manual intervention and improving the consistency and speed of compliance activities. * **Comprehensive Audit Trails Required:** Even advanced automation requires comprehensive, immutable audit trails to provide regulatory bodies with clear evidence of data changes, user actions, and system processes. * **Role-Based Access Control:** Access to the safety database must be strictly controlled and defined by user roles, ensuring that data security and integrity are maintained and that only authorized personnel can perform specific functions. * **Regulatory Evolution Requires System Evolution:** The safety database must be treated as a "living system" that requires continuous adaptation and updating to remain compliant with evolving global and local regulations (e.g., changes from the FDA or EMA). * **Validation and Documentation are Key:** Any planned updates or changes to the database configuration must be preceded by careful risk identification, followed by thorough validation of the changes, and complete documentation of the entire process to ensure inspection readiness. * **Local vs. Global Requirements:** Configuration must balance global reporting standards (like E2BR3) with local regulatory nuances, including region-specific timelines, seriousness criteria, and submission formats. Tools/Resources Mentioned: * Argus Safety (Safety Database System) * Veeva Vault Safety (Safety Database System) Key Concepts: * **Pharmacovigilance (PV):** The science and activities relating to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problem. * **E2BR3:** The current international standard for the electronic transmission of Individual Case Safety Reports (ICSRs), defining the data elements and format required for regulatory submissions. * **Audit Trails:** Secure, computer-generated, time-stamped electronic records that record the history of system operations, including data entry, modification, or deletion, critical for 21 CFR Part 11 and GxP compliance. * **Risk-Based Validation:** A methodology for validating computerized systems where the scope and rigor of validation activities are determined by the potential risk the system poses to patient safety, data integrity, and regulatory compliance.

The Tokio-Marine HCC 2025 Stop-Loss Report: 5 Key Takeaways
Self-Funded
@SelfFunded
Aug 15, 2025
This video provides an in-depth exploration of the Tokio Marine HCC 2025 Stop-Loss Report, breaking down five key takeaways that illuminate the current hardening market for stop-loss insurance. The speaker, a self-proclaimed "stop-loss geek," aims to equip listeners with critical knowledge to navigate projected medical cost increases of 9-10% in 2025 and increasingly challenging renewals. The analysis delves into the specific trends driving these shifts, offering a granular look at the frequency and severity of large claims, the surprising cost implications of COBRA claimants, the prevalence of policy "lasers," and the crucial concept of loss ratio maturity. The presentation systematically unpacks the report's findings, starting with diagnostic categories, differentiating between those most frequently leading to large claims (e.g., neoplasms/cancer, cardiovascular diseases) and those driving the highest severity costs (e.g., transplants, congenital anomalies, blood diseases like hemophilia). It then transitions to a striking analysis of large claims over time, revealing a dramatic increase in the frequency of multi-million dollar claims, which have necessitated a redefinition of what constitutes a "catastrophic" claim. The speaker attributes some of these trends to the impact of the Affordable Care Act (ACA), particularly the removal of lifetime and annual maximums, and the rise of expensive treatments like gene therapies. Further insights are provided on the financial burden posed by COBRA claimants, who are shown to be significantly more likely to incur large claims than active employees, prompting a discussion on potential solutions for employers. The video also clarifies the concept of loss ratio maturity, explaining how a policy's loss ratio at different points in its term can predict its final outcome, which is highly relevant for renewal negotiations. Finally, the analysis covers the frequency of "lasers" (specific deductibles for named individuals) on stop-loss policies, comparing market averages with Tokio Marine's own practices and discussing the influence of "no new laser" provisions and captive insurance models. The overarching message is that the market is experiencing a significant rebound and hardening, partly due to underwriters misinterpreting the post-COVID cost plateau as normal rather than a unique event, leading to artificially suppressed rates that are now correcting sharply. Key Takeaways: * **Hardening Stop-Loss Market:** The stop-loss market is experiencing significant hardening, with renewals becoming tougher due to projected medical cost increases of 9-10% in 2025, a trend expected to continue for several years. * **Dramatic Increase in Large Claims:** Claims exceeding $2 million are now 1,250% more frequent than in 2013, while $500,000 claims are up 250%. This necessitates a redefinition of "catastrophic" claims, with $1 million no longer considered exceptionally large and $2 million becoming the new benchmark. * **Key Diagnostic Cost Drivers (Frequency):** Neoplasms and cancer consistently rank as the most frequent large claim categories, followed by cardiovascular, musculoskeletal, and digestive diseases. Nervous system diseases (e.g., Multiple Sclerosis, with expensive medications) have recently moved into the top five. * **Key Diagnostic Cost Drivers (Severity):** Transplants remain the most expensive category, with congenital anomalies (e.g., malformations, premature babies in NICU) and blood diseases (e.g., hemophilia, often requiring costly gene therapies or ongoing medication) also being major drivers of severe costs. * **Impact of Medical Advances:** Advances in medical technology, particularly gene therapies, are significant contributors to the rising cost and duration of large claims, as they often involve ongoing, expensive treatments rather than cures. * **High Cost of COBRA Claimants:** Individuals electing COBRA are substantially more expensive on average than active employees. They are 13.5 times more likely to have a $200,000 claim, 14.1 times more likely for a $500,000 claim, and 15.5 times more likely for a $1 million claim, highlighting a significant risk for plan sponsors. * **Employer Solutions for COBRA:** Employers should consider offering solutions or guidance to COBRA-eligible members to explore open market options. This can help mitigate the significant financial risk associated with retaining high-cost claimants on the employer's plan. * **Loss Ratio Maturity for Renewals:** Understanding loss ratio maturity is crucial for renewals. The further into a policy period (e.g., 9 months), the more predictable the final loss ratio becomes, allowing stop-loss carriers to price renewals more accurately and potentially less conservatively. * **Laser Frequency Trends:** On average, about one in four policies in the market has a "laser" (a specific deductible for a named individual), with an average of one laser per policy. However, carriers like Tokio Marine HCC demonstrate significantly lower laser frequency, suggesting better data, larger case sizes, or higher risk tolerance. * **Influence of "No New Laser" Provisions:** The growth of "no new laser" provisions and captive insurance models is likely contributing to a slight decrease in the overall number of policies that have lasers placed on them. * **Post-COVID Rebound Effect:** The current market hardening is exacerbated by stop-loss underwriters having viewed the cost plateau in 2021-2022 (due to COVID-related deferral of care) as normal credible experience rather than a unique event. This artificially suppressed rates, leading to a more severe "boomerang" effect now. * **Contributing Factors to Rising Costs:** Beyond medical inflation and large claims, other factors include supply and demand dynamics, lower Medicare reimbursements shifting costs to the commercial sector, and the post-COVID rebound in healthcare utilization. Key Concepts: * **Stop-Loss:** Insurance that protects self-funded employers from catastrophic claims. * **Laser:** A specific, higher deductible applied to one or more named individuals on a stop-loss policy, carving out their exposure. * **Loss Ratio Maturity:** The concept that a policy's loss ratio at different points in its term can predict its final loss ratio, with predictability increasing as the policy matures. * **Medical Inflation/Trend:** The rate at which healthcare costs are increasing over time. * **Leverage Trend:** The amplified impact of underlying medical trend on stop-loss costs, as stop-loss covers only the portion of claims above a certain deductible. * **ACA (Affordable Care Act):** Legislation that impacted healthcare coverage, including medical loss ratio (MLR) requirements and the removal of lifetime and annual maximums, contributing to changes in large claim frequency. Tools/Resources Mentioned: * Tokio Marine HCC 2025 Stop-Loss Report (linked in the video description) * Paro (speaker's partner)