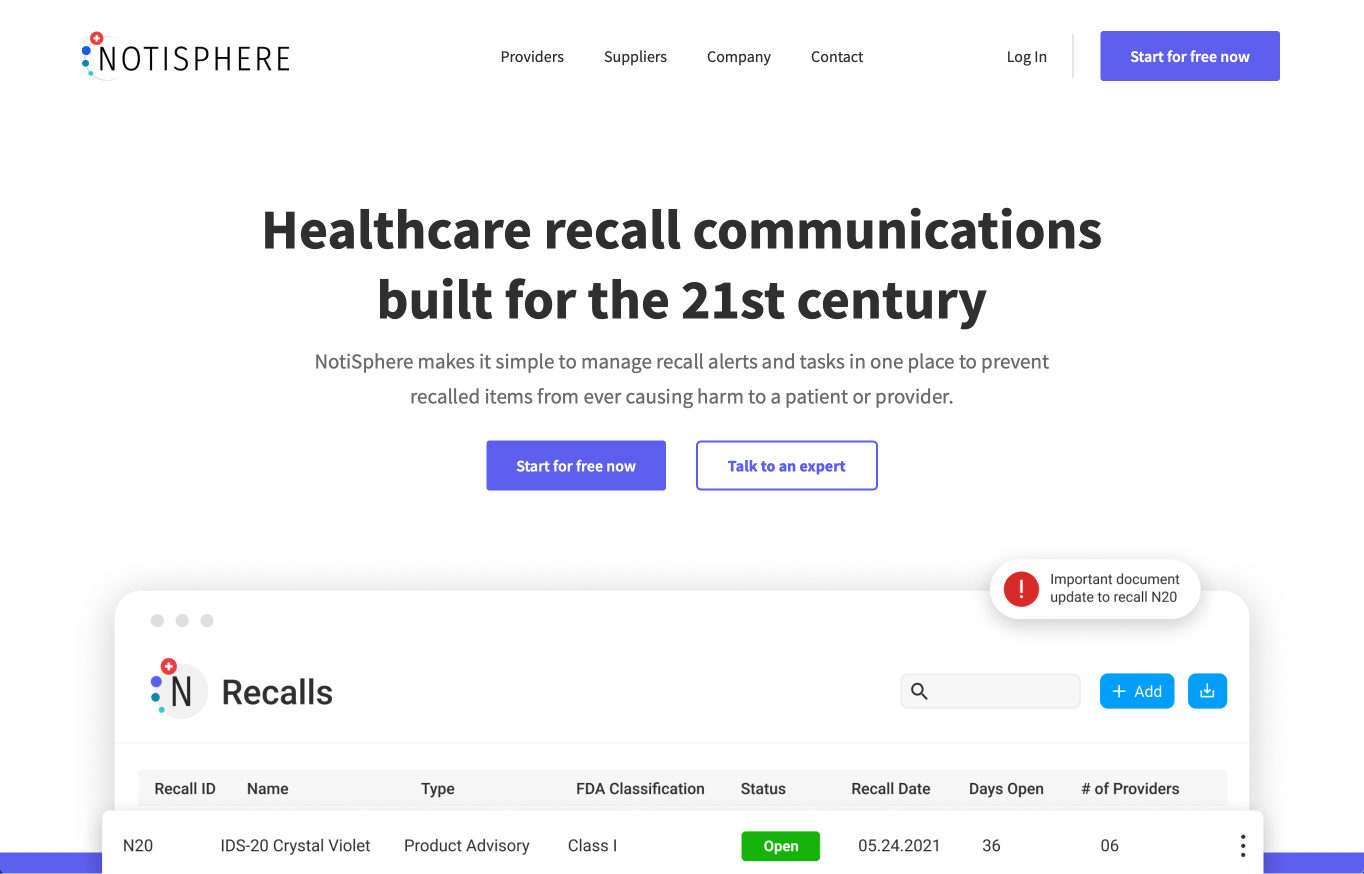
InVita UDITracker is a trusted, UDI-compliant software solution designed for hospitals and surgery centers to manage the complex lifecycle of both tissue and non-biologic implants, from receipt to the point of use. Used by over 700 organizations, the platform automates critical, time-consuming processes to improve compliance, reduce staff overload, and optimize inventory management.
Key Features and Capabilities:
- Full Implant Lifecycle Management: Tracks tissue, orthopedic, and cardiovascular implants throughout their entire lifecycle, including receipt, inventory, patient use, and explants.
- Automated Recall Management: Integrates with the FDA Recall Database, Electronic Health Records (EHRs), and device manufacturers' databases to automatically match recalls to affected patients and inventory locations, expediting the process and ensuring patient safety.
- Inventory Optimization: Provides real-time visibility to avoid stockouts, reduce loss from expiration, and optimize inventory with data-driven insights.
- Integrated Bill-Only Management: Automates the documentation, submission, and reconciliation of supplier representative sales order forms and purchase order requests.
- Compliance & Audit Readiness: Offers built-in compliance safeguards, maintains a two-way audit trail, and helps meet accreditation standards set by The Joint Commission (JCAHO), FDA, and DNV.
- Barcode & RFID Support: Functions with 1D and 2D barcode scanning and integrates with RFID cabinets for end-to-end tracking and temperature control monitoring.
Target Users and Use Cases: UDITracker is primarily used by clinical, materials management, and supply chain departments in hospitals and health systems. Its main use cases include tissue and implant tracking in the Operating Room (OR) and interventional suites, ensuring regulatory compliance, and automating the warranty claims and joint replacement revision processes.