Videos
Browse videos by topic
All Videos
Showing 73-96 of 142 videos

RIM Implementation in times of IDMP - Asphalion Webinar
Asphalion
/@Asphalion.
Jul 26, 2022
This video explores the critical intersection of Regulatory Information Management (RIM) system implementation and the ongoing transition to ISO Identification of Medicinal Products (IDMP) standards. The speaker details the current uncertainties surrounding IDMP, the phased rollout of EMA's SPOR project (including PMS and the DADI web form), and the profound impact these changes have on the pharmaceutical industry. A central theme is how RIM systems serve as essential tools to navigate IDMP compliance, emphasizing the need for digitalization, robust data governance, and strategic data migration during implementation. Key Takeaways: * **IDMP as a Digitalization Catalyst:** ISO IDMP is presented not merely as a regulatory requirement but as a significant driver for comprehensive digitalization and the establishment of strong data governance frameworks within pharmaceutical companies, necessitating standardized and interoperable data. * **RIM Systems are Core for IDMP Compliance:** Regulatory Information Management (RIM) systems are highlighted as indispensable for IDMP compliance, facilitating efficient data collection, organization, and communication. Future RIM systems are expected to directly support data submission to EMA's PMS via fire messages, evolving beyond current xEVMPD processes. * **Data Quality and Governance are Foundational:** Successful IDMP compliance and RIM implementation are contingent upon high-quality, harmonized data. Companies must prioritize data cleaning, enrichment, and mapping, integrating IDMP requirements from the initial planning stages of any RIM project. * **Navigating Phased IDMP Rollout Complexity:** The EMA's phased implementation of IDMP through the DADI web form (starting with Centrally Authorized Products (CAPs), then Nationally Authorized Products (NAPs)) introduces a complex transitional period. Organizations must plan to manage the coexistence of legacy and new systems and processes effectively. * **Strategic Implementation is Crucial:** Regardless of company size or portfolio, a well-defined strategy for IDMP compliance and RIM implementation is vital. This involves a thorough assessment of existing data structures, internal processes, technological capabilities, and future expectations to select the most compatible RIM system and implementation approach.

Veeva post Implementation.
Prana Life Sciences
/@pranalifesciences1
Jul 21, 2022
This video explores strategies for optimizing Veeva Vault post-implementation support within the life sciences industry. The speaker, a technical expert from Prana Life Sciences, discusses common challenges and presents practical solutions for improving efficiency, reducing costs, and ensuring compliance in regulated GxP environments. Key themes include the automation of user and access management, streamlining configuration support, and enhancing clinical operations processes within Veeva CTMS and eTMF. The presentation emphasizes a continuous improvement approach, leveraging technology to address manual bottlenecks and accelerate project delivery. Key Takeaways: * **Automated User & Access Management:** Significant efficiency gains can be achieved by automating Veeva user creation and deactivation. This can be done by integrating with identity management systems (e.g., SalePoint, Azure Active Directory) or using custom development (Python, Java SDKs), drastically reducing manual effort and ensuring timely access/deactivation. * **Streamlined Configuration Support:** Improving configuration support involves proactive categorization of incoming tickets to quickly identify potential change requests. Initiating change management and validation (CSV) processes earlier, along with engaging users through quick Proof-of-Concepts (POCs) during design, can reduce lead times and enhance SLA adherence. * **Enhanced Clinical Operations Efficiency:** Automation can substantially reduce the resource burden in clinical support. Specific examples include automating user provisioning and access changes for studies/sites within Veeva CTMS and eTMF, as well as automating the generation of reports for milestones and sites, leading to significant resource optimization (e.g., reducing support from 2 full-time resources to 0.5). * **Continuous Improvement in Regulated Environments:** Adopting a continuous improvement mindset, supported by regular reporting on support activities and Service Level Agreements (SLAs), is crucial for maintaining high efficiency and customer satisfaction in GxP-regulated settings, ensuring processes are consistently optimized. * **Addressing Deactivation Complexities:** While automating user deactivation can present challenges, particularly with active workflows, these can be managed by generating reports of users with active workflows, allowing for targeted reassignment of tasks before full deactivation, thus maintaining process integrity while still benefiting from automation.

An Industry Ontology for the Identification of Medicinal Products (IDMP)
Biomedical Ontology World
/@biomedicalontologyworld
Jul 18, 2022
This video introduces the Pistoia Alliance's IDMP Ontology project, which addresses the critical challenge of inconsistent interpretations of the ISO Identification of Medicinal Products (IDMP) standards within the pharmaceutical industry. Currently, IDMP standards are published as PDFs, leading to varied implementations across companies and regulatory agencies, hindering automated data processing, quality, and semantic interoperability. The project aims to augment these standards with a formal ontology, creating a semantically aligned and governed data model that acts as a "connecting bridge" for data interpretation across internal departments, suppliers, and regulators. The speaker emphasizes the importance of contextualizing complex concepts like "active moiety" to accommodate diverse scientific and regulatory perspectives, ensuring accurate representation while allowing for different expert views. The ontology is being developed collaboratively with major pharma companies and validated against real-world data from sources like FDA's GSRS, demonstrating its ability to answer specific competency questions and facilitate cross-organizational data integration for improved drug safety, innovation, and operational efficiency. Key Takeaways: * **Semantic Interoperability for Regulatory Compliance:** The IDMP Ontology directly tackles the lack of semantic alignment in pharmaceutical product data, a crucial step for achieving consistent regulatory compliance (FDA, EMA) and enabling automated data processing across the life sciences ecosystem. * **Ontologies as Foundational Data Bridges:** The project highlights the strategic value of ontologies in creating a unified "connecting bridge" for data interpretation, essential for harmonizing information flows within and between pharma companies, suppliers, and regulatory bodies. * **Contextualized Data Modeling for Complex Concepts:** The approach to modeling concepts like "active moiety" with contextual roles (e.g., regulatory, scientific, patent exclusivity) demonstrates a sophisticated method for managing inherent ambiguities and diverse expert perspectives in pharmaceutical data. This advanced data modeling is critical for developing robust custom software and AI solutions. * **Industry-Wide Collaborative Standard Setting:** The Pistoia Alliance's collaborative model, involving leading pharma companies (Bayer, Merck, GSK, Novartis, J&J) and regulatory agencies (FDA, EMA), underscores the industry's commitment to developing shared data standards that drive collective innovation, efficiency, and patient safety. * **Real-World Validation for Practical Application:** The project's emphasis on testing the ontology with actual data (e.g., FDA GSRS, pharma company data) and specific use cases ensures its practical applicability and demonstrates tangible value for solving real-world data integration and interpretation challenges, a key aspect of delivering effective consulting and software development.

TMF Reference Model Training Part 2
TMF Reference Model
/@TMFReferenceModel
Jun 20, 2022
This video provides a comprehensive training on the Trial Master File (TMF) Reference Model, defining its purpose, structure, and application in clinical trials. Speakers Chole Xi Van and Donna Dorzinski, both members of the TMF Reference Model Steering Committee, explain how the model standardizes TMF content, naming, structure, and metadata, expanding upon ICH GCP Chapter 8 and EU Regulation 536/2014. The discussion covers the model's 11 functional zones, the classification of artifacts and sub-artifacts, and how the model facilitates consistent filing, rapid retrieval, and regulatory compliance. It also highlights the benefits of implementing the model, such as reduced customization, simplified CRO engagement, and improved integration of TMFs. Key Takeaways: * **Standardized Regulatory Compliance:** The TMF Reference Model provides a critical framework for clinical trial documentation, ensuring compliance with regulations like EU Regulation 536/2014 and ICH GCP. Its four-fold purpose (standard content, naming, structure, and metadata) is essential for demonstrating the conduct and data quality of a trial during audits and inspections. * **Structured for Efficiency and Retrieval:** The model's detailed organization into 11 functional zones, sections, artifacts, and sub-artifacts, complete with definitions and ICH codes, enables consistent filing and rapid retrieval of documents. This structured approach is vital for operational efficiency and audit readiness in clinical operations. * **Industry Consensus and Reduced Customization:** As an industry-driven initiative, the TMF Reference Model offers a consistent interpretation of TMF requirements, reducing ambiguity and avoiding scope creep. Its adoption helps limit company-specific customizations, aligning organizations with broader industry standards and simplifying collaboration with CROs. * **Foundation for Data Management and Integration:** By standardizing content and metadata, the model facilitates the exchange of information across the industry and simplifies the integration of multiple TMFs into a single, unified structure. This structured approach is foundational for robust data engineering and business intelligence initiatives within clinical data management. * **Enabling Digital Transformation:** While not explicitly mentioning AI, the emphasis on standardized structure, naming, and metadata within the TMF Reference Model creates an ideal environment for the implementation of electronic TMF (eTMF) systems and future AI/LLM solutions.

TMF QC Discussion
Power of Work
/@powerofwork6914
May 10, 2022
This video provides a practical guide to Trial Master File (TMF) Quality Control (QC), focusing on the meticulous review process essential for regulatory compliance and inspection readiness in clinical trials. The speaker details the critical steps involved in QC, from verifying document legibility and completeness to ensuring accurate metadata entry within systems like Veeva eTMF. The discussion highlights the significance of adhering to Good Clinical Practices (GCP) principles, specifically ALCOA-C, and identifies common documentation errors such as missing signatures or incorrect dates. Furthermore, the video delves into the interdependencies of various clinical trial documents, including FDA Form 1572, financial disclosure forms, training logs for Site Initiation Visits (SIVs), monitoring reports, and investigational product shipment forms, underscoring the collaborative effort required from study teams, monitors, and clinical trial assistants (CTAs) to maintain an accurate and up-to-date TMF. Key Takeaways: * **TMF QC is paramount for regulatory inspection readiness:** The primary purpose of TMF QC is to ensure documents meet regulatory requirements (e.g., FDA) and are prepared for audits, preventing delays in drug approval. * **Adherence to ALCOA-C principles is fundamental:** TMF documents must be Attributable, Legible, Contemporaneous, Original, Accurate, and Complete to ensure data integrity and compliance, a core tenet of Good Clinical Practices (GCP). * **Comprehensive document review involves multiple checks:** QC encompasses verifying legibility, page inclusion, required signatures, correct dates, accurate document titles, classification, linking, and expiry status, often by comparing physical documents to system metadata. * **Veeva eTMF is a key platform for TMF management:** The video explicitly references the Veeva eTMF home screen and metadata sections, indicating its widespread use in the industry and the importance of understanding its functionalities for effective TMF QC. * **Interconnectedness of clinical trial documentation:** Various documents like monitoring reports, follow-up letters, training logs (especially for Site Initiation Visits), and investigational product shipment forms are interdependent and must be reviewed as a complete packet to ensure study integrity. * **Site training and turnover impact TMF quality:** Effective training during Site Initiation Visits (SIVs) and proactive management of site staff turnover are crucial for maintaining high-quality documentation and preventing errors due to lack of knowledge or experience. * **The Clinical Trial Assistant (CTA) role is foundational:** CTAs play a critical role in managing and QCing TMF documents, highlighting the need for robust processes and training for entry-level positions in clinical operations.

VEEVA CRM Training – VEEVA CRM Online Training – (VEEVA CRM Certification Tips)– VEEVA CRM Course
MaxMunus Training
/@maxmunustraining
Apr 19, 2022
This video provides an overview of Veeva CRM training, emphasizing its specific design and application within the life sciences industry. The speaker details a comprehensive course content covering Veeva CRM architecture, administration, data security, application configuration, custom object creation, and various functionalities like call management, cycle plans, sample management, data loading, and reporting. The discussion also highlights the benefits of Veeva CRM for life sciences, such as coordinating planning, boosting productivity while ensuring compliance, and driving continuous improvement. Furthermore, the video addresses career opportunities and certification for Veeva CRM professionals, showcasing significant global demand for these skills. Key Takeaways: * **Life Sciences Specialization:** Veeva CRM is presented as the premier multi-channel CRM solution explicitly designed for the unique needs of the life sciences industry, including drug companies, medical institutions, and healthcare organizations, enabling integrated 360-degree planning and execution. * **Extensive Configuration Capabilities:** The detailed course content underscores the depth of Veeva CRM's configurability, from foundational elements like system architecture and admin console to advanced features such as custom objects, data access security, call management, sample management, and territory management. * **Compliance and Productivity Focus:** A core benefit highlighted is Veeva CRM's ability to enhance commercial operations by improving collaboration, optimizing sales utilization, and ensuring field teams engage compliantly with healthcare providers * **High Market Demand for Expertise:** The video demonstrates a robust global job market for Veeva CRM professionals, with numerous openings and competitive compensation, indicating a critical need for specialized skills in implementation, configuration, and ongoing management of the platform. * **Implementation-Level Training:** The training emphasizes a practical, implementation, and configuration-level approach, aiming to make participants "project-ready" from day one. This suggests that hands-on, deep technical expertise is paramount for successful Veeva CRM deployment and optimization within client environments.

VEEVA VAULT Online Training Explained By Expert: Everything You Need to Know
MaxMunus Training
/@maxmunustraining
Apr 19, 2022
This video provides an overview of Veeva Vault training, highlighting its importance as a cloud-based enterprise content management platform specifically designed for the biological sciences. It emphasizes Veeva Vault's role in providing a single source of truth for content and data, thereby minimizing complexity and enhancing business agility within life sciences organizations. The training covers a comprehensive range of topics, from basic end-user functions like document and binder management to advanced administrative operations such as configuring document types, workflows, and dynamic access controls. The video also details Veeva's certification programs and underscores the significant global job opportunities available for Veeva Vault professionals, citing major pharmaceutical and life sciences companies as key employers. Key Takeaways: * **Veeva Vault as a Core Life Sciences Platform:** Veeva Vault is presented as an indispensable cloud enterprise content management platform for biological sciences, crucial for streamlining regulatory processes, data management, and ensuring compliance by providing a unified source of truth. * **Comprehensive Functional Scope:** The training curriculum covers a wide array of Veeva Vault functionalities, from fundamental document and binder operations to intricate system administration, including document type hierarchies, workflow creation, and dynamic access control, indicating its deep capabilities for managing complex industry content. * **High Market Demand for Expertise:** There is a robust global job market for Veeva Vault professionals across various roles (e.g., Veeva Vault Lead, Senior Manager Veeva CRM, Analyst, Developer), with significant opportunities in major pharmaceutical and biotech companies, highlighting the value of this specialized skill set. * **Integration within the Veeva Ecosystem:** The mention of "Veeva Products Overview" and "Veeva CRM Certified White Belt qualification" suggests that Veeva Vault is an integral part of a broader Veeva suite, implying that expertise in Vault is complementary to other Veeva solutions like CRM. * **Focus on Implementation and Configuration:** The training emphasizes "implementation and configuration level" learning, indicating a practical, hands-on approach designed to make participants "project ready" and capable of deploying and managing Veeva Vault effectively from day one. * **Regulatory Compliance and Data Integrity:** The platform's design for "biological sciences" to "minimize complexity" and serve as a "unique source of truth" directly addresses critical industry needs for regulatory compliance, data integrity, and efficient information governance, aligning with stringent industry standards.

eTMF System Specific Training (Veeva Vault)
Power of Work
/@powerofwork6914
Apr 13, 2022
This video provides specific training on the Veeva Vault eTMF (electronic Trial Master File) system, focusing on its features and functionalities for managing clinical trial documentation within the life sciences industry. The speaker, from Power of Work, guides viewers through navigating the system, applying filters, viewing documents, and critically, generating reports. The discussion highlights the transition from paper-based TMFs to eTMFs, emphasizing the significant advantages Veeva Vault offers in terms of regulatory compliance, data visibility, collaboration, and security. It also touches upon the system's role in supporting TMF specialists and its broader integration within the Veeva ecosystem. Key Takeaways: * **Veeva Vault as a Prevalent eTMF Solution:** Veeva Vault is presented as a widely adopted, comprehensive, cloud-based eTMF platform, particularly suited for large pharmaceutical companies due to its extensive features and the robust IT support required for its maintenance and updates. * **Ensuring Regulatory Compliance:** The video underscores the critical role of eTMF systems like Veeva Vault in maintaining regulatory compliance, specifically mentioning 21 CFR Part 11, electronic signatures, and audit trails. It emphasizes that a complete and accurate TMF is essential for successful FDA and other regulatory agency inspections. * **Enhanced Data Visibility and Reporting Capabilities:** Veeva Vault significantly improves operational efficiency by offering advanced filtering and reporting functions. These allow users to gain real-time insights into TMF completeness, track document processing, and monitor team performance, which is crucial for proactive management and audit readiness. * **Secure Collaboration and Audit Trails:** The system facilitates secure external collaboration, enabling multiple stakeholders to access and contribute to the TMF with defined roles and permissions. Its robust audit trail functionality ensures accountability and traceability for all document interactions, enhancing data integrity. * **Strategic Importance of Metadata Management:** The training highlights that a key aspect of TMF quality control involves cross-referencing document content with its associated metadata within the system, stressing the necessity for accurate data entry and consistency for effective document retrieval and reporting. * **Integration within the Life Sciences Technology Landscape:** The video notes Veeva Vault's evolving integration capabilities, including its use as a Clinical Trial Management System (CTMS), indicating its broader role and interconnectedness within the suite of technology solutions utilized in clinical research.

eTMF Systems Introduction
Power of Work
/@powerofwork6914
Apr 1, 2022
This video provides a comprehensive introduction to eTMF (electronic Trial Master File) systems, detailing their evolution from paper-based documentation to digital platforms in clinical research. The speaker emphasizes the critical role of eTMFs in managing the extensive documentation generated during clinical trials, highlighting their importance for regulatory compliance, particularly during FDA audits. The content covers the historical context, the necessity for digital transformation due to increasing trial complexity, and the numerous advantages of eTMF systems, including enhanced document retrieval, real-time tracking, robust quality control processes, improved reporting, SOP compliance, cost savings, and seamless collaboration between sponsor companies and Contract Research Organizations (CROs). The video also touches upon specific eTMF systems, notably mentioning Veeva Vault, and discusses practical aspects of document management, metadata entry, and filing structures within these systems. * **Operational Efficiency and Data Integrity:** The digital nature of eTMFs significantly improves operational efficiency through features like real-time tracking, timestamping, and multi-step quality control, ensuring data integrity and reducing the risks associated with manual, paper-based processes. * **Data Management and Reporting Capabilities:** eTMF systems enable sophisticated data management through structured metadata and robust reporting tools, allowing for better organization, retrieval, and analysis of clinical trial documents * **Addressing Industry Skill Gaps:** The video highlights a significant industry demand for professionals with technical eTMF system skills combined with clinical research knowledge, indicating a potential market for specialized consulting, training, or AI-powered assistance for these roles. * **Facilitating Collaboration in Clinical Ecosystems:** eTMFs are crucial for fostering efficient collaboration among diverse stakeholders in clinical trials, including sponsors, CROs, and sites, by providing controlled, shared access to critical documentation.

IDMP in a capsule Tutorial
UNICOM
/@UNICOM-IDMP
Mar 24, 2022
This. The emphasis on patient safety and pharmacovigilance further underscores its relevance to medical affairs and regulatory compliance departments within their target market. This tutorial provides a comprehensive overview of the Identification of Medicinal Products (IDMP) standards, highlighting their crucial role in ensuring global medication safety. It explains how the ISO IDMP standards, though inconsistently implemented, are designed to work throughout a medicinal product's life cycle, from development and production to utilization and outcome assessment. Through illustrative stories, the video demonstrates how IDMP helps prevent adverse drug events, facilitates safe substitutions across different countries, curbs falsified medicines, and enhances global pharmacovigilance. A central theme is the concept of Medicinal Product Dictionaries (MPDs) as national repositories for comprehensive product information, linked globally by the Pharmaceutical Product Identifier (PHP ID), enabling seamless data navigation and personalized patient care through integration with personal health data. Key Takeaways: * **Standardized Global Identification is Critical:** Inconsistent identification of medicinal products across countries poses significant patient safety risks. IDMP provides a standardized framework (substance, dose form, strength, medicinal product, package) to ensure globally unique identification, crucial for safe healthcare and preventing errors. * **Medicinal Product Dictionaries (MPDs) as Central Data Hubs:** MPDs are vital national repositories that consolidate comprehensive product information (regulatory, scientific, pricing, dosage guidance). These dictionaries are the foundation for all information needed by hospitals, doctors, and pharmacies for safe prescribing and dispensing, and are accessed through various healthcare IT systems. * **The PHP ID Enables Cross-Border Data Linkage:** The Pharmaceutical Product Identifier (PHP ID) is a critical global identifier that allows for the seamless navigation and comparison of equivalent medicinal products across different national MPDs. This capability is essential for facilitating safe substitutions for patients traveling internationally and for aggregating global pharmacovigilance data. * **IDMP Supports Personalized Patient Safety:** By integrating IDMP-compliant product data from MPDs with individual patient health data (e.g., International Patient Summary - IPS), intelligent applications can provide real-time, personalized alerts for allergies, intolerances, and potential drug-drug interactions, significantly enhancing medication safety at the point of care. * **Enhanced Global Pharmacovigilance:** IDMP-compliant reporting, which includes the PHP ID and other identifiers, streamlines the aggregation and analysis of adverse drug event data globally. This enables faster identification of safety patterns, quicker responses (e.g., product recalls, updated warnings), and proactive risk mitigation across populations. * **Call for Industry-Wide Implementation:** The full benefits of IDMP, including improved medication safety and public health outcomes, can only be realized through widespread and consistent implementation by pharmaceutical companies, regulators, and IT solution providers across the entire medicinal product life cycle.

CTMS Oversight Deep Dive Demo
Veeva Systems Inc
/@VeevaSystems
Mar 22, 2022
This video provides a deep dive into Veeva Vault CTMS, showcasing its capabilities for comprehensive clinical trial oversight. It highlights how the platform facilitates effective collaboration, streamlines negotiations with CRO partners, and ensures regulatory compliance. The demonstration covers integrated study management, from initial planning and site activation to ongoing monitoring, risk assessment, and issue resolution. A central theme is the seamless integration between CTMS and TMF components, ensuring all clinical activities and documentation are managed within a unified, compliant system. The video also emphasizes robust data tracking, automated processes, and advanced reporting for real-time insights into study progress, site performance, and CRO oversight. Key Takeaways: * **Integrated Clinical Operations & Compliance:** Veeva Vault CTMS provides a unified platform for managing clinical trials, integrating CTMS and TMF functionalities to ensure seamless document management, regulatory compliance (e.g., 21 CFR Part 11 for signatures, automatic TMF artifact creation), and inspection readiness. * **Enhanced CRO and Vendor Oversight:** The system offers robust tools for managing and collaborating with CROs, including a global personnel directory for access control, automated notifications for CRO-submitted data (e.g., trip reports), dedicated issue logging against CROs, and dashboards for tracking vendor performance and issue resolution times.ai to offer AI-powered enhancements for predictive CRO performance, automated contract compliance checks, or intelligent issue triage. * **Proactive Risk Management & Issue Resolution:** The platform supports templatized risk assessments with automated scoring and integrated mitigation plans. It also provides comprehensive issue management capabilities, allowing for logging, assignment (including to CROs), tracking of resolution times, and trending analysis of quality findings and protocol deviations.ai could leverage AI/LLMs to enhance risk prediction, suggest optimal mitigation strategies, or automate the generation of issue summaries. * **Data-Driven Decision Making & Business Intelligence:** Extensive reporting and dashboard features provide real-time visibility into study progress, enrollment metrics, site performance, and TMF completeness. This enables data-driven decision-making, identification of at-risk milestones, and comparative analysis of CRO performance. * **Streamlined Workflow Automation:** The system automates several critical workflows, such as subject data calculation for actuals, automatic completion of milestones, generation of risk assessment documents, and creation of Part 11 compliant trip reports, significantly reducing manual effort and enhancing efficiency.
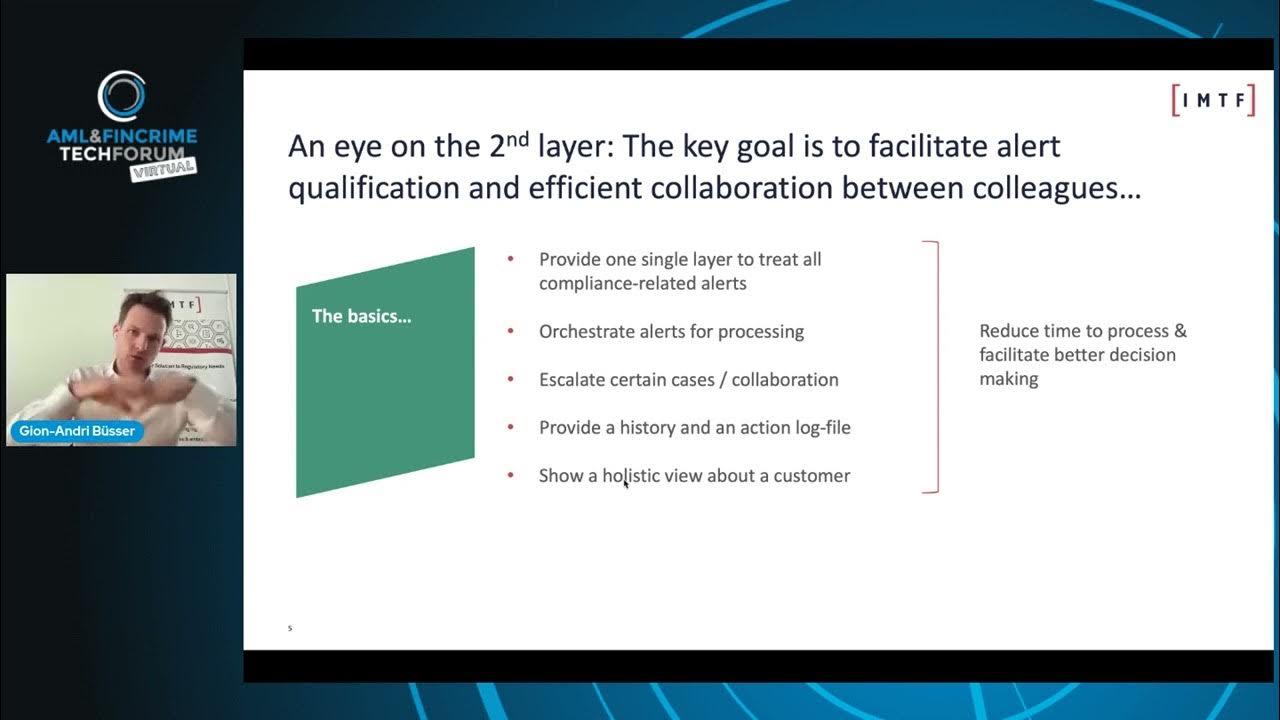
Benefits of a Two Layer Regulatory Intelligence Platform
IMTF - Excellence in RegTech Solutions
/@imtf-excellenceinregtech
Mar 11, 2022
The speaker, Gion-Andri Büsser of IMTF, discusses the critical need for modern technology in compliance, driven by exploding costs of compliance and even higher costs of non-compliance. He introduces a "two-layer regulatory intelligence platform" concept, emphasizing an orchestration layer that centralizes the treatment of compliance alerts, automates processes, fosters collaboration, and provides a holistic view of data. The video highlights how advanced technologies, including machine learning and data science, can be leveraged within this architecture to significantly reduce manual work, improve efficiency, and enhance risk management. Key Takeaways: * **Ecosystem Approach to Compliance Technology:** The video advocates for viewing compliance technology as an integrated ecosystem rather than disparate, siloed software packages. This platform approach is crucial for comprehensive data utilization and decision-making. * **The Power of the Orchestration Layer (Layer 2):** A dedicated orchestration layer is presented as essential for consolidating and processing all compliance alerts (e.g., AML, fraud, sanctions), enabling flexible process automation, fostering collaboration across teams, and maintaining accurate, regulator-ready audit trails. * **AI/ML for Enhanced Efficiency and Risk Reduction:** The "magic" of the orchestration layer lies in its ability to leverage AI and machine learning for advanced functions such as intelligent auto-closure of routine alerts, pre-processing to highlight historical patterns, and more granular, accurate risk rating based on comprehensive customer history. * **Strategic IT and Change Management Benefits:** Implementing an orchestration layer provides significant IT flexibility, allowing organizations to introduce modern tools and interfaces without immediately replacing existing, potentially valuable, legacy detection systems (Layer 1). This enables a phased modernization approach, reducing disruption and risk. * **Continuous Adaptation to Evolving Threats:** The speaker underscores that financial crime and compliance challenges are an "ever-ending battle," with criminal behaviors constantly evolving (e.g., due to pandemics, crypto). This necessitates adaptive, forward-looking technology to anticipate and combat new threats.g., GxP, 21 CFR Part 11, FDA/EMA regulations).

Veeva Systems (VEEV) Stock Analysis and Intrinsic Value | Buy Now or Wait?
Andrew Finance
/@andrewfinance5351
Mar 11, 2022
This video provides a detailed financial and technical analysis of Veeva Systems (VEEV) stock, assessing its intrinsic value using Discounted Cash Flow (DCF) and Earnings Per Share (EPS) models under various performance scenarios. It delves into Veeva's business strengths, risks, and future growth prospects, ultimately providing an investment recommendation. The analysis highlights Veeva's strong market position as a cloud-computing leader in the pharmaceutical and life sciences industries, its robust financial health, and its strategic expansion into other regulated sectors. Key Takeaways: * **Veeva's Dominance in Life Sciences:** Veeva Systems is firmly established as a leading cloud-computing provider for the pharmaceutical and life sciences industries, characterized by strong subscription revenue, high switching costs, and a strategic relationship with Salesforce, which limits competition in its core niche. * **Robust Financial Health and Growth Outlook:** The company demonstrates excellent profitability with a 23% profit margin, 11% return on assets, and 15% return on equity, coupled with a strong balance sheet. It is projected to achieve significant revenue growth (14% yearly) and EPS growth (10% yearly) over the next five years, indicating a healthy and expanding ecosystem for Veeva-centric solutions. * **Strategic Market Expansion:** Veeva has begun extending its content and data management solutions beyond life sciences into other regulated sectors such as consumer goods, chemicals, and cosmetics, signaling potential future growth avenues and broader applicability of its platform. * **Competitive Landscape and Operational Risks:** Despite its specialized market, Veeva faces competition from major enterprise software vendors like Oracle and Microsoft. Key risks include potential slowdowns in near-term growth due to labor pressures or challenges in closing large deals, customer churn, commercial execution issues, and litigation. * **Market Valuation Perspective:** The analysis concludes that Veeva stock is currently overvalued, recommending investors wait for signs of reversal or a price drop below $130 before considering an investment.

TMF & Quality Control
Power of Work
/@powerofwork6914
Mar 4, 2022
This video provides a comprehensive overview of Trial Master File (TMF) quality control (QC) within clinical trials, emphasizing its critical role in regulatory compliance and drug approval. The speaker details how TMF documentation serves as proof of a drug's safety and efficacy, ensuring adherence to Good Clinical Practices (GCP) and ICH guidelines. The discussion covers the practical aspects of TMF management, including the use of electronic TMF (eTMF) systems like Veeva, the importance of TMF maps and plans for document organization, and the meticulous process of performing QC checks. These checks involve verifying document completeness, correct filing, metadata accuracy, and the presence of required signatures, all while safeguarding patient privacy. The video highlights the challenges of managing vast amounts of documentation and the necessity for rapid document retrieval during audits and inspections. Key Takeaways: * **TMF as a Regulatory Cornerstone:** The TMF is indispensable for demonstrating regulatory compliance (FDA, ICH, GCP) in clinical trials, serving as the primary evidence for drug safety, efficacy, and patient protection. * **Operational Efficiency through Organization:** Effective TMF management, utilizing tools like the TMF Reference Model and company-specific TMF plans, is crucial for maintaining organized documentation and enabling rapid retrieval, which is vital during audits and inspections. * **Meticulous Quality Control is Paramount:** A rigorous QC process for TMF documents is essential, focusing on completeness, accurate metadata, correct filing, signature verification, and the absence of Protected Health Information (PHI) to ensure audit-readiness. * **Veeva's Role in eTMF Management:** The video explicitly mentions Veeva as a common system for managing eTMFs, underscoring the prevalence of specialized software in handling the complexities of clinical trial documentation. * **Challenges and Opportunities for Automation:** The manual and time-intensive nature of TMF QC, coupled with the high volume and complexity of documents, presents significant challenges that could be addressed through advanced automation and AI solutions for classification, metadata extraction, and compliance checks. * **The "Gatekeeper" Role of TMF Processors:** Document specialists act as critical "gatekeepers," ensuring the integrity and accuracy of the TMF, identifying discrepancies, and clearly documenting findings for resolution by document owners, thereby upholding the overall quality of clinical trial records.

Smart Classifier: Bulk Classification & Data Segregation
NextLabs
/@NextLabsInc
Jan 13, 2022
This video introduces NextLabs’ Smart Classifier, a tool designed for bulk classification, organization, and protection of documents in shared folders and SharePoint sites. It emphasizes the importance of a strong data governance framework for data safety and productivity, showcasing how the Smart Classifier streamlines data management processes. The system operates through file watchers, content extractors, and rule engines to automatically classify files based on their content or metadata. The demonstration highlights two key use cases: bulk classification of existing documents (e.g., ITAR/EAR) to enforce attribute-based access control (ABAC) policies, and automated data segregation, where classified documents are moved to designated "system of record" folders. The solution ensures sensitive data is secured with fine-grained access controls and digital rights protection, preventing unauthorized disclosure. * **Scalable Compliance and Security:** The ability to classify and protect documents in bulk, using rules based on content or metadata, addresses the challenge of managing vast volumes of sensitive data. This scalability is essential for pharmaceutical companies dealing with large datasets from R&D, clinical trials, and commercial operations, ensuring consistent application of security and compliance policies. * **Enhanced Data Quality and Management:** By automating classification and segregation, the solution improves data quality and streamlines data management processes. * **Fine-Grained Access Control (ABAC) for Sensitive Data:** The integration of attribute-based access control (ABAC) allows for dynamic, fine-grained control over sensitive documents, preventing unauthorized disclosure. This is crucial for protecting intellectual property, patient data, and other confidential information within the life sciences sector * **Addressing Legacy Data Challenges:** The video highlights the challenge of classifying existing, unclassified files. Smart Classifier's bulk classification feature provides a solution for bringing legacy data into compliance, a common pain point for established pharmaceutical companies.
![[Webinar] Automating Clinical Trial Master File Migration & Information Extraction](https://i.ytimg.com/vi/IchcgkDDdtw/hqdefault.jpg?sqp=-oaymwEcCNACELwBSFXyq4qpAw4IARUAAIhCGAFwAcABBg==&rs=AOn4CLD8QvOyM_6WqAniWDO0HGPWtHJEqw)
[Webinar] Automating Clinical Trial Master File Migration & Information Extraction
John Snow Labs – Healthcare AI Company
/@JohnSnowLabs
Jan 13, 2022
This video explores the significant challenges and an AI-powered solution for automating Clinical Trial Master File (TMF) migration and information extraction within pharmaceutical companies. It highlights that TMF migration is a complex, labor-intensive process due to the massive volume of unstructured documents (scanned, handwritten), lack of standardization, and bespoke rules, rendering manual methods and traditional Robotic Process Automation (RPA) ineffective. The presented solution leverages Natural Language Processing (NLP) and Artificial Intelligence, including advanced OCR and machine learning, to classify documents, extract critical metadata, and ensure data accuracy and regulatory compliance. A detailed case study with Novartis demonstrates the real-world application, showcasing substantial reductions in manual effort and migration timelines while adhering to stringent industry standards like GxP and GAMP 5. Key Takeaways: * **TMF Migration Complexity:** Clinical Trial Master File migration is inherently complex due to the lack of content standardization, high volume of unstructured data (scanned, handwritten), and non-explicit, bespoke rules, making manual or basic automation approaches impractical. * **AI-Driven Efficiency & Accuracy:** AI and NLP-based systems, incorporating advanced OCR and machine learning, can achieve an 80% reduction in manual labor and migration timelines for TMFs, significantly improving efficiency and accuracy in metadata extraction and document classification. * **Critical Solution Components:** Successful AI solutions for TMF migration rely on meticulously defined annotation guidelines, sophisticated post-processing rules (which can boost accuracy by 30-40% by handling ambiguities like multiple dates or names), and machine learning-driven false positive detectors to ensure data quality. * **Regulatory Compliance & Enterprise Readiness:** Solutions must be designed for enterprise deployment, offering scalability (e.g., Apache Spark-based), on-premise (air-gapped) security, and rigorous validation for regulatory compliance, including GxP and GAMP 5. * **Collaborative Expertise:** Effective implementation requires a multi-disciplinary team combining deep AI/data science expertise with extensive subject matter knowledge in TMF, document management, IT security, quality, and validation from both the solution provider and the client.

Top 10 TMF Specialist Interview Questions||Trial Master File||TMF||Clinical Research
Vikas Singh
/@VikasSinghPharmalive
Dec 7, 2021
This video delves into the critical aspects of Trial Master File (TMF) management, a cornerstone of regulatory compliance and clinical operations within the pharmaceutical and life sciences industries. This video explores the essential role of a TMF Specialist and addresses frequently asked interview questions related to the Trial Master File (TMF) in clinical research. The speaker defines TMF as a collection of essential documents that demonstrate a clinical trial's compliance with regulatory requirements and ICH GCP. Key topics include the types of documents found in a TMF, the advantages of electronic TMF (eTMF) over physical TMF, the importance of TMF completeness and quality control, and the principles of Good Documentation Practice (GDP), specifically ALCOA (Attributable, Legible, Contemporaneous, Original, Accurate). The video also covers TMF archiving, retention periods, and the critical handling of Personally Identifiable Information (PII) within the TMF. Key Takeaways: * **TMF as a Regulatory Compliance Foundation:** The Trial Master File (TMF) is presented as the central repository for all essential documents of a clinical trial, crucial for demonstrating adherence to regulatory requirements (ICH GCP, FDA, EMA) and ensuring audit readiness. * **Benefits of Electronic TMF (eTMF):** The video highlights significant advantages of eTMF over traditional paper-based systems, including real-time access, improved document location, enhanced quality control, and better preparedness for regulatory inspections. * **Criticality of Good Documentation Practices (ALCOA):** The ALCOA principles (Attributable, Legible, Contemporaneous, Original, Accurate) are emphasized as fundamental for maintaining data integrity, reliability, and regulatory acceptance of all TMF documents. * **Proactive TMF Management for Audit Readiness:** Continuous completeness checks, quality control, and proper archiving are essential for maintaining an inspection-ready TMF, minimizing risks and ensuring smooth processes during regulatory audits. * **Data Privacy and PII Protection:** The video underscores the paramount importance of strict handling and protection of Personally Identifiable Information (PII) within the TMF, highlighting the need for robust data governance and compliance with privacy regulations.

RegTalks about Regulatory Information Management Systems (RIMS)
Asphalion
/@Asphalion.
Nov 29, 2021
This RegTalks episode, featuring Lidia Canovas of Asphalion and Udo Griem of Körber Pharma Software, provides an in-depth discussion on Regulatory Information Management Systems (RIMS) within the pharmaceutical and biotech industries. The speakers emphasize the critical role of RIMS in managing medicinal product data, particularly in anticipation of the complex transition to ISO IDMP standards. Griem defines RIMS through three core pillars: a solid, data-oriented database serving as a single source of truth for product master data, a robust workflow engine to manage submissions and approval processes, and capabilities for structured data submissions to authorities. He advocates for a data-oriented approach over traditional document-driven methods to ensure data quality and consistency. Key drivers for RIMS implementation include the impending IDMP mandate, the industry's shift towards structured data, competitive pressures for faster time-to-market, and the need for organizational process optimization. RIMS are shown to be beneficial for companies of all sizes and product portfolios, fostering a culture of quality and improving cross-departmental collaboration. While regulatory affairs is the primary user, RIMS serve as a central hub, supporting cross-functional processes across clinical, ERP, safety/pharmacovigilance, MES, and label artwork management. A significant emerging trend highlighted is the connection of RIMS data to data lakes for future evaluation with artificial intelligence. The discussion also underscores the importance of experienced implementation partners who can bridge the gap between software solutions and organizational needs, ensuring successful deployment and continuous improvement. Körber's AI Manager RIMS aims to minimize compliance risks, enhance efficiency, enable teamwork, and reduce costs, with IDMP compliance being a high priority in its development roadmap. **Key Takeaways:** * **RIMS are Foundational for Regulatory Compliance and Efficiency:** RIMS are critical for managing complex regulatory data, streamlining submissions, and ensuring compliance with evolving standards like IDMP, which is rapidly approaching and will significantly increase data complexity. * **Data-Oriented Approach is Paramount for RIMS:** A consistent, data-oriented design with a well-organized database is considered superior to document-driven approaches, ensuring high data quality, consistency, and adaptability for future regulatory requirements. * **RIMS Drive Cross-Functional Integration and Quality Culture:** Beyond regulatory affairs, RIMS act as a central hub, connecting and supporting various departments (e.g., clinical, ERP, safety, MES, QA) to improve collaboration, reduce error rates, and shorten response times across the pharmaceutical value chain. * **AI Integration is an Emerging Trend for RIMS Data:** There is a growing focus on connecting RIMS data to data lakes, specifically for evaluation with artificial intelligence, indicating a future direction for leveraging regulatory information for advanced insights and automation. * **Implementation Partners are Crucial for Successful RIMS Adoption:** Experienced partners with deep regulatory and product knowledge are vital for guiding companies through organizational and process changes, customizing solutions, and ensuring successful integration and continuous improvement. * **RIMS Benefits Extend to All Company Sizes and Diverse Portfolios:** While historically adopted by large multinationals, RIMS are now essential for small and mid-tier firms, including startups, to effectively build and scale regulatory organizations and manage diverse product portfolios beyond human medicine.

RegTalks about Regulatory Information Management Systems (RIMS)
Asphalion
/@Asphalion.
Nov 5, 2021
This video explores the critical role of Regulatory Information Management Systems (RIMS) within the pharmaceutical industry, particularly in light of the upcoming transition from XEVMPD to the more complex ISO IDMP standard. Featuring insights from Veeva's Director of Strategy for Vault RIM, Katrin Spaepen, the discussion highlights the drivers for RIMS adoption, essential capabilities of future systems, and the multi-departmental nature of their use. The speakers emphasize that RIMS are key to managing vast amounts of regulatory data, ensuring compliance, and optimizing operations for medicinal products. Key Takeaways: * **Strategic Importance of RIMS:** RIMS are fundamental for data management, operational efficiency, and maintaining regulatory compliance in the pharmaceutical industry, especially with the impending, more complex ISO IDMP standard. * **Drivers for RIMS Adoption:** Companies adopt RIMS for three primary reasons: driving enterprise-wide digital transformation, achieving process efficiencies and cost optimizations in regulatory domains, or ensuring compliance (often driven by mandates like IDMP). These drivers influence the scope and approach to implementation. * **Core Capabilities of Future RIMS:** Effective RIMS must offer native support for both data and documents, ensure synchronization between them, feature an open architecture for data exchange with non-RIM systems, provide a complete view of license data across the enterprise, and embed real-time reporting and business intelligence dashboards. * **Multi-Departmental Utility:** RIMS are not solely for regulatory affairs; they serve as an enterprise-wide solution involving various departments such as regulatory operations, CMC writers, medical writers, manufacturing, quality control, and safety, particularly in processes like post-approval variation management. * **Crucial Role of Implementation Partners:** Successful RIMS implementation necessitates strong partnerships for process re-engineering, organizational impact assessment, change management, gap analysis, and defining key performance indicators (KPIs) and user requirements (URS). * **Veeva's Market Presence:** Veeva's Vault RIM suite (encompassing submissions, archive, publishing, and registration) is positioned as a leading end-to-end solution supporting the full regulatory process, indicating its significance in the industry.

CTMS & eTMF implementation – Flex Databases & FGK CRO case study
Flex Databases
/@Flexdatabases
Nov 1, 2021
This video presents a case study on the successful implementation of Flex Databases' Clinical Trial Management System (CTMS) and electronic Trial Master File (eTMF) for FGK CRO, a full-service contract research organization. The discussion highlights FGK CRO's objectives to standardize and simplify clinical trial management across various phases and sponsors, aiming for increased cost-effectiveness and quality. The video details the project scope, a rapid implementation timeline (5.5 weeks vs. 8 weeks estimated), and emphasizes the critical role of client involvement in achieving a smooth and accelerated deployment. Key decision factors for FGK CRO included the system's clear structure, intuitive handling, flexible modules (e.g., investigator payments), offline capabilities for CRAs, and the competence of the Flex Databases team. Key Takeaways: * **Active Market for eClinical Solutions:** CROs and pharmaceutical companies are actively investing in and implementing eClinical platforms like CTMS and eTMF to enhance the efficiency, standardization, and quality of clinical trial operations. * **Importance of Implementation Expertise:** Successful and expedited software implementation is a critical value proposition, with client-vendor collaboration and high client involvement being crucial for achieving faster-than-expected deployment times. * **Client-Centric System Selection:** Key criteria for selecting eClinical systems include intuitive user interfaces, clear structure, specific functional advantages (e.g., offline access for CRAs, flexible payment modules), and the vendor's team competence. * **Foundation for Advanced Data Solutions:** The successful deployment of core eClinical systems like CTMS and eTMF creates a structured data environment, which serves as an ideal foundation for integrating advanced data engineering, business intelligence, and AI/LLM solutions to further optimize clinical operations and regulatory compliance. * **Focus on Regulated Environments:** The context of clinical trials and eClinical systems inherently involves strict regulatory compliance

Webinar: Webinars in Pharma. Are you ready to integrate Veeva and Salesforce Marketing journeys?
showerthinking
/@showerthinking
Oct 27, 2021
This video explores the strategic integration of webinar platforms with Veeva and Salesforce Marketing Cloud to create comprehensive, automated marketing journeys within the pharmaceutical and healthcare industries. The speakers advocate for a shift from viewing webinars as isolated tactics to leveraging them as integral touchpoints within a broader cross-channel customer experience, aimed at enriching HCP knowledge, generating leads, and optimizing digital marketing strategies. The discussion covers best practices for platform selection, data synchronization, consent management, HCP identification, and empowering sales representatives with real-time visibility and actionable insights. A detailed case study highlights a multinational pharma company's successful implementation, showcasing one-click registration, LinkedIn ad integration, and robust analytics using Google Tag Manager and DataRama to measure engagement via Key Educational Messages (KEMs). Key Takeaways: * **Strategic Shift for Webinars in Pharma:** Webinars should evolve from isolated events to integrated components of a larger, automated marketing journey, focusing on improving the HCP customer experience and enriching customer data. * **Seamless Cross-Channel Integration is Crucial:** Effective digital marketing in pharma requires a seamless integration between webinar platforms, Salesforce Marketing Cloud, and Veeva CRM, enabling journeys that can be triggered by reps (Veeva Approved Email) and digital channels (Marketing Cloud emails, social media, advertising). * **Data Synchronization and HCP Identification:** A core challenge and opportunity is to accurately identify HCPs across platforms, synchronize data (especially Veeva identifiers), and manage multi-channel consents within Veeva to personalize interactions and avoid redundant data requests (e.g., one-click registration). * **Empowering Sales Reps with Visibility and Actionable Insights:** Integrated solutions should provide reps with real-time visibility into HCP engagement on their Veeva timeline and suggest "next best actions" based on journey interactions, enhancing rep-HCP interactions. * **Comprehensive Analytics for Campaign Performance:** Beyond basic platform analytics, a robust solution integrates data from Veeva, Salesforce Marketing Cloud, webinar platforms, and advertising channels (like LinkedIn) into a business intelligence tool (e.g., DataRama) to measure campaign performance, track engagement (e.g., KEMs), and inform future strategies. * **Regulatory Compliance and Content Access:** Solutions must ensure that content access is restricted to verified HCPs, often through approval processes based on Veeva identifiers, and that all consent management adheres to industry regulations. * **Optimizing User Experience and Conversion:** Leveraging features like one-click registration, deep linking for content access, and native social media forms (e.g., LinkedIn forms) significantly improves the user experience and conversion rates for HCPs.

Quality and Quality Systems (QMS and TQM) | Operations Management | Quality Control
Data Driven Management
/@datadrivenmanagement6230
Oct 20, 2021
This video provides a foundational understanding of quality and quality management systems, specifically discussing Total Quality Management (TQM) and Quality Management Systems (QMS). It begins by defining quality through various perspectives, such as fitness for use, conformance to specifications, and customer satisfaction, and then elaborates on key dimensions of quality including performance, reliability, durability, and serviceability. The discussion further breaks down quality into three aspects: quality of design, quality of conformance (manufacturing), and quality of performance (in use), highlighting their interconnectedness. The video then differentiates and relates TQM and QMS, presenting TQM as a company-wide philosophy focused on continuous improvement and customer satisfaction, while QMS is described as a system of standards, like ISO 9000, designed to help organizations meet customer and regulatory requirements. It outlines the principles of QMS, such as customer focus, leadership, and a process approach, and details the elements and outcomes of TQM, including waste elimination, defect reduction, and innovation. Key Takeaways: * **Foundational Quality Definitions:** Quality is multifaceted, defined by fitness for use, conformance to specifications, and customer requirements, with dimensions spanning performance, reliability, and serviceability, which are critical considerations for any product or service in regulated industries. * **QMS for Regulatory Adherence:** Quality Management Systems (QMS), particularly those based on ISO standards (e.g., ISO 9000, ISO 15189), provide structured frameworks for organizations to consistently meet both customer expectations and stringent regulatory requirements, which is essential for compliance in pharmaceutical and life sciences. * **TQM for Continuous Operational Excellence:** Total Quality Management (TQM) promotes a holistic, company-wide commitment to continuous improvement, employee involvement, and customer focus, aligning with the goal of optimizing operations and fostering long-term efficiency within complex enterprise environments. * **Integrated Quality Lifecycle:** Quality encompasses the entire lifecycle from design (quality of design) through implementation and manufacturing (quality of conformance) to actual use (quality of performance), emphasizing the need for quality considerations at every stage of solution development and deployment. * **Tangible Benefits of Quality Systems:** Implementing effective QMS and TQM leads to significant outcomes such as waste elimination, reduction of defects and variations, and fostering innovation, all of which directly contribute to improved efficiency and compliance in highly regulated sectors.

RegTalks about Regulatory Information Management Systems (RIMS)
Asphalion
/@Asphalion.
Oct 1, 2021
This video directly addresses Regulatory Information Management Systems (RIMS) within the pharmaceutical and life sciences industries, a core area for companies seeking to optimize operations and maintain regulatory compliance. **Summary:** The "RegTalks" video, featuring Lidia Canovas from Asphalion and Frank Dickert from EXTEDO, provides an in-depth exploration of Regulatory Information Management Systems (RIMS) and their increasing importance in the pharmaceutical and life sciences sectors. The speakers define RIMS as comprehensive solutions for master product data management, regulatory activity tracking, and integrated document management, serving as a crucial "single source of truth." A central theme is the significant industry transition from xEVMPD to the complex ISO IDMP standard, which necessitates robust RIMS to handle increased data transmission and regulatory compliance. The discussion highlights how RIMS enhance operational efficiency by replacing fragmented manual systems, improve impact assessment capabilities, and are applicable across various regulated fields beyond human medicines, including animal health and medical devices. Furthermore, the video emphasizes that RIMS benefit a wide array of departments beyond regulatory affairs, such as quality, clinical, manufacturing, and supply chain, and stresses the indispensable role of experienced implementation partners in successfully deploying these complex systems. **Key Takeaways:** * **RIMS as a Foundational Compliance System:** Regulatory Information Management Systems (RIMS) are critical enterprise solutions for the life sciences, providing a "single source of truth" for master product data, regulatory activities, and integrated document management, essential for ensuring regulatory compliance and operational efficiency. * **IDMP Transition Drives RIMS Adoption:** The impending transition to the ISO IDMP standard is a major catalyst for RIMS implementation, as it significantly increases data complexity and submission requirements, necessitating sophisticated systems to manage and submit data effectively, often integrated with eCTD submissions. * **Broad Organizational Impact:** RIMS are not exclusive to regulatory affairs but serve a wide range of departments across a life sciences company, including quality, clinical, manufacturing, marketing, and supply chain, by providing centralized access to regulatory information, thereby improving cross-functional decision-making and impact assessment. * **Beyond Human Medicines:** The utility of RIMS extends beyond human pharmaceuticals to other regulated sectors such as animal health, consumer health, and medical devices (e.g., for IVDR/MDR compliance), indicating a broader market need for robust regulatory data management solutions. * **Criticality of Implementation Expertise:** Successful RIMS deployment, especially in the context of IDMP compliance, requires knowledgeable external implementation partners who can provide technical support, guide necessary process changes, and leverage deep industry experience to ensure optimal system configuration and user adoption.

What is TMF Reference model||DIA||Trial master file||Clinical Research
Vikas Singh
/@VikasSinghPharmalive
Jul 4, 2021
This video explores the Trial Master File (TMF) Reference Model, detailing its historical context, purpose, and structure. The speaker explains the evolution from paper-based clinical trial documentation, which was time-consuming for audits, to electronic Trial Master Files (eTMFs). The video highlights the challenge of inconsistencies arising from different companies and countries using varied eTMF systems, which the TMF Reference Model was created to address. As a supported initiative of the DIA, the model provides a unified interpretation of regulations and best practices for TMFs, serving as a reference tool for document location, keywords, and quality checks. It outlines the model's four main areas (Content, Location, Keywords, Quality Check) and various sections (e.g., Trial Management, Regulatory, Site Management), emphasizing its role in ensuring proper filing and management of essential clinical trial documents for regulatory compliance and operational efficiency. Key Takeaways: * **Standardization for Compliance:** The TMF Reference Model is crucial for standardizing clinical trial documentation, providing a unified interpretation of regulations and best practices to ensure consistency and facilitate regulatory audits, which is vital for FDA and EMA compliance. * **Evolution to eTMF:** The industry's transition from inefficient paper-based TMFs to electronic TMFs (eTMFs) was driven by the need for more efficient auditing, with the TMF Reference Model addressing initial inconsistencies in eTMF implementations. * **Structured Document Management:** The model defines specific sections and provides detailed information (e.g., Artifact ID, purpose, retention, language) for categorizing and managing essential documents, enabling precise location and quality control within clinical operations. * **Operational Efficiency:** By standardizing document filing and naming conventions, the TMF Reference Model significantly enhances the efficiency of clinical operations, data management, and compliance tracking