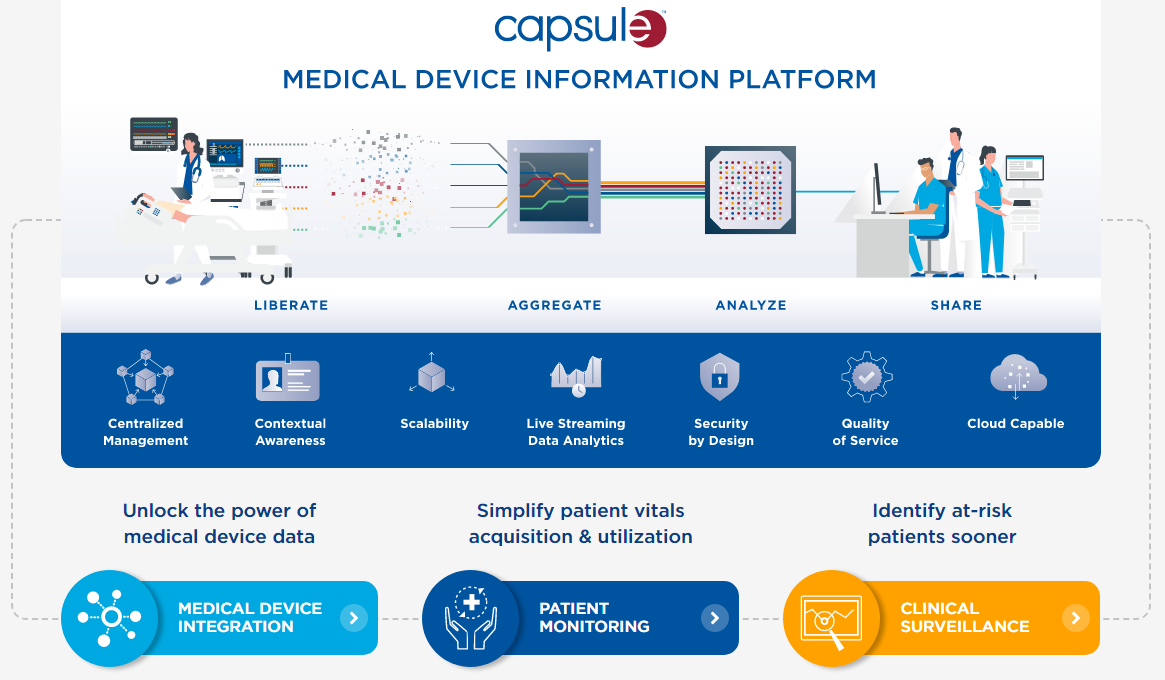
Capsule Technologies, now part of Philips, provides the Medical Device Information Platform (MDIP), a comprehensive, vendor-neutral solution for liberating, aggregating, analyzing, and sharing clinical data from connected medical devices across a healthcare enterprise. The platform is designed to transform complex, live-streaming medical device data into context-rich, actionable insights to support proactive care delivery.
Key Capabilities:
- Medical Device Integration (MDI): The platform connects to over 1,200 unique medical device models from over 100 different manufacturers using a comprehensive Device Driver Interface (DDI) library. This vendor-neutral approach ensures interoperability and a single, standardized interface for all device data.
- Clinical Surveillance (Capsule Surveillance): This solution continuously analyzes live-streaming data using a Smart Rules engine to detect early signs of patient deterioration (e.g., respiratory depression, tachyarrhythmia). It facilitates proactive care by sending precise, condition-specific notifications to care teams, helping to reduce alarm fatigue.
- Clinical Information System (Philips IntelliSpace Critical Care and Anesthesia - ICCA): A clinical decision support and documentation solution for critical and perioperative care environments that centralizes and streamlines visual data.
- Retrospective Analytics (Clinical Insights Manager): A service-oriented solution that synthesizes high-fidelity clinical data for retrospective analysis, reporting, and research to support evidence-based care and quality improvement initiatives.
Target Users & Use Cases:
The software is primarily targeted at hospitals and healthcare organizations (IT/Biomed, Clinicians, C-suite). It is used to enhance patient safety, streamline clinical workflows (e.g., automated charting with Capsule Chart Xpress), and provide comprehensive patient views across critical care, non-critical care, operating rooms, and virtual care settings (e.g., Tele-ICU).